Neurochemical and Neurophysiologic Effects of Needle Insertion: Clinical Implications
Michael J. Butler
Philip J. Siddall
This chapter surveys the effects of needle insertion per se, with an emphasis on controlled animal studies of the neurochemical and neurophysiologic consequences of transdermal needling, particularly analgesia. Human research on the effects of transcutaneous needle insertion, and analgesia in particular, is complicated by the challenge of establishing an appropriate control intervention to isolate the objective effects of needle insertion from other factors such as the placebo response. The placebo response, in turn linked to other factors such as expectation, may play a significant role in the clinical consequences of needling (1,2). Psychological influences upon analgesia, and the placebo effect specifically, are discussed in detail by Katz and Melzack, and Finniss and Benedetti, respectively, in the next two chapters of this volume.
The effects of needle insertion per se during regional anesthetic techniques are difficult if not impossible to ascertain, as active and control groups typically undergo needle insertion at identical skin entry points followed by injection of saline or inactive placebo through the needle (or no solution, in instances of sham injection). Therefore, this chapter emphasizes the substantial basic and clinical evidence amassed during studies of acupuncture. The authors of this chapter were both trained in clinical acupuncture in Beijing, China, then in subsequent years proceeded with initial skepticism to apply this technique to treat a range of disorders. Our practical experience indicated apparently beneficial effects but also noxious side effects that may be of relevance to needle insertion by the regional anesthetist. Controlled animal research on acupuncture analgesia reveals diverse and widespread neurochemical and neurophysiologic effects of needle insertion, beyond simply placebo and nocebo analgesic responses.
Acupuncture is defined as “pricking with a needle, specifically the insertion of needles into living tissues for remedial purposes, other than for the injection of drugs” (3). To prick is to “pierce slightly, puncture, or perforate, especially with a fine or sharp point,” and to pierce is to “penetrate as a sharp pointed instrument does, or to make a hole, opening, or tunnel into or through (something), to bore through or perforate, make (a hole, etc.) by pricking or stabbing with a sharp pointed instrument” (3).
These definitions are important as, too easily, acupuncture is believed to be inseparable from traditional Chinese medical teachings and Oriental medical philosophy that describe presumptive lines of energy (meridians), empirically specifying needle insertion at precise points, and often specifying the type of needle stimulation to be used, either manual or thermal (moxibustion). Yet, one need not practice traditional Chinese medicine to deliver acupuncture treatment.
Most of the animal research summarized below is from Professor Han Ji-Sheng’s laboratory at Beijing Medical College. Over many years, this research program elucidated the neurochemical basis of acupuncture analgesia using rigorous experimental and analytical methodology. Although initially these studies were published in Chinese scientific and medical journals in the Chinese language, steadily increasing numbers of publications in this area have appeared in peer-reviewed, English-language scientific and medical journals. We accept that animal research is not devoid of a placebo effect, nor, in relation to pain, is the confounding effect of stress-induced analgesia. Likewise recognizing progressive advances in trial design and experimental rigor of clinical trials in acupuncture, we will adopt a “best available evidence” approach to our survey of relevant clinical trials.
Mechanisms Underlying Needling Effects
Early Acupuncture Research
The animal research program into acupuncture analgesia (AA) was initiated by Han and colleagues in an effort to understand the results of a human observational study (4,5). The effect of acupuncture on the pain threshold was explored in 66 healthy volunteers and 22 nonstimulated controls, as well as smaller numbers of patients with paraplegia and hemiplegia following strokes (n = 13 and 12, respectively). A modified potassium iontophoresis method delivering progressively increasing anodal currents through skin electrodes was employed to produce graded nociceptive stimuli. Eight points, distributed over various sites on the body including the forehead and back, and paired points on the chest, abdomen, and legs, received nociceptive stimuli. Pain thresholds were measured every 10 minutes over 80 minutes, and expressed as a percentage change from baseline in the intensity of electrical current tolerated. Acupuncture (unilateral needle insertion into the thenar eminence at the site of maximal thickness) was given over 15 minutes. A statistically significant increase in pain threshold occurred at all measurement sites, maximal at about 40 minutes and declining rapidly (half-life 16.2 +/- 1.9 minutes)
when acupuncture ceased. No significant rise in pain threshold occurred in the unstimulated control group.
when acupuncture ceased. No significant rise in pain threshold occurred in the unstimulated control group.
The slow increases in pain threshold induced by acupuncture, and the production of a bilateral analgesic effect following unilateral needling, suggested the hypothesis that acupuncture evoked the release of certain chemical substances within the body that in turn produced the analgesic effect. Acupuncture analgesia was blocked by local infiltration of procaine at the site of thenar stimulation, and also was absent when the insensate extremities of paraplegic or hemiplegic patients were stimulated. These observations indicated that afferent impulses to the central nervous system (CNS) were necessary for optimal AA, and led to the decision to focus on the chemistry of the CNS in future planned controlled animal research.
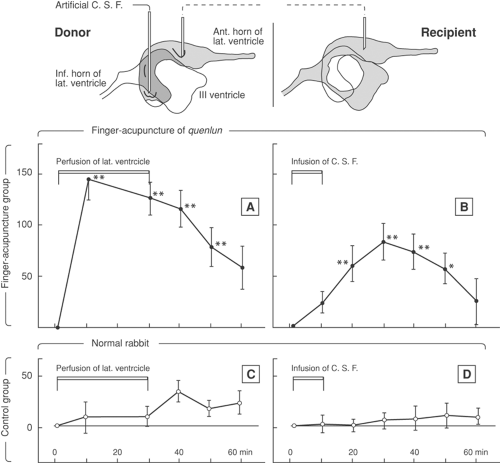
A cerebroventricular perfusion model was developed in rabbits (6) exposed to “finger acupuncture” (i.e., repetitive manual stimulation with the fingers) of the Achilles tendon near its attachment. In fact, either needling of a specific point just distal to the knee or repetitive finger pressure at the Achilles tendon insertion brought about a similar increase in thermal pain threshold of the debristled skin around the nostrils and mouth, indicated by a prolonged latency of head withdrawal. Stainless steel cannulae were stereotactically inserted under general anesthesia with their tips in the lateral cerebral ventricle, thereby allowing withdrawal or infusion of cerebrospinal fluid (CSF). Connection between a donor and recipient rabbit was made with polyethylene tubing between the cannulae, thereby allowing transfer of CSF from donor to recipient rabbits. The tubing was perfused with ice-cold saline to reduce catabolism. In the experimental model, the donor was given finger acupuncture for 30 minutes, and CSF was transferred from donor to the unstimulated recipient. A statistically significant increase in the pain threshold of the recipient occurred, although not as marked as in the donor. This effect was compared with the control experiment of CSF transfer from an unstimulated donor to an unstimulated recipient (Fig. 34-1).
This observation suggested that chemicals with analgesic effect were produced in the brain of the donor rabbit in the course of acupuncture stimulation. Intracerebroventricular (ICV) injection of reserpine enhanced and prolonged the analgesic effect of finger acupuncture. This effect was reversed by ICV replacement of norepinephrine/noradrenaline or dopamine, suggesting that augmentation of finger acupuncture analgesia by reserpine may be related to its effect of depleting monoamines in the brain. Intracerebroventricular injection of atropine significantly reduced the effect of finger acupuncture analgesia, presumably by blocking the muscarinic effect of acetylcholine in the brain. Morphine analgesia and finger acupuncture analgesia were compared: ICV injection of reserpine blocked the analgesic effect of morphine while augmenting that of finger acupuncture analgesia. Intracerebroventricular injection of atropine also blocked morphine analgesia, while substantially reducing finger acupuncture analgesia, suggesting differences between the underlying mechanisms of morphine analgesia and finger acupuncture analgesia.
From 1973, Han and colleagues studied the role of central neurotransmitters in AA, the main questions being: Does needle insertion (acupuncture) bring about any change in the content and turnover rate of central neurotransmitters? Could the effect of AA be modified by selective alteration of these neurotransmitters? For each type of neurotransmitter, the nature of its effect on AA was determined both qualitatively (i.e., facilitatory or inhibitory) and quantitatively (magnitude of effect, dose response, and time course). The site of action in the CNS was also studied.
Animal Models of Acupuncture Analgesia
Two major animal models of AA were studied:
The rabbit finger acupuncture model just described, with finger acupuncture to the Achilles tendon attachment and nociceptive (radiant heat) stimuli to the snout or tail, measuring the avoidance response latency (ARL) (7)
The rat tail flick model, with radiant heat stimuli either to the lower leg or the tail and tail flick as the response. The nociceptive threshold is measured as tail flick latency (TFL) in seconds between the application of the heat stimulus and withdrawal of the tail (8).
Neurochemical Basis of Acupuncture Analgesia
A seminal review by Han, co-authored with Terenius of the University of Uppsala, Sweden, was published in 1982 (9). This comprehensive introduction to the complexity of the neurochemical basis of AA emphasized controlled animal research and provided extensive (n = 193) references. This large number of references was provided to raise awareness of much of the prior research that had been published in Chinese- and Japanese-language journals not easily accessible to the Western scientific community. The emphasis on research paper selection in this chapter will be on peer-reviewed English-language publications.
Serotonin
To evaluate the effect of ascending serotonergic pathways, 5-6-dihydroxytryptamine (5-6DHT), a chemical depleter of neuronal serotonin (5-hydroxytryptamine, 5HT), was injected into the medial forebrain bundles of rats. A significant reduction in the magnitude of AA occurred, together with selective lowering of forebrain 5HT content, implying that ascending serotonergic fibers may play an important part in mediating the effect of AA (10).
In a study in the rabbit, cinanserin, which blocks the post synaptic 5HT receptor, was injected into the periaqueductal gray (PAG) bilaterally at a dose sufficiently low not to affect basal nociceptive threshold in either electroacupuncture (EA) or morphine analgesia (MA) experiments. The analgesic effect induced by EA and morphine was markedly reduced, suggesting involvement of serotonergic synaptic transmission in the PAG area for EA and morphine analgesia. The site specificity of this effect was also suggested by the observations that bilateral injection was more effective than unilateral injection, and that injection beyond the area of the PAG was ineffective.
In a later study in the rat, an immunocytochemical double-staining technique was used to investigate the effects of EA on the expression of c-fos oncogene in the serotonergic neurons in the nucleus raphe dorsalis (NRD) (11). The number of c-fos positive serotonergic cells in the NRD increased significantly after EA stimulation. Further studies in the rat implicated 5HT receptor subtypes in spinal antinociception (12) and characterized the relevant 5HT receptor subtypes mediating supraspinal μ-opioid–induced analgesia (13).
Repeated or prolonged EA results in a gradual decrease in the analgesic effect in the rat (14). Such EA tolerance and its cross-tolerance to MA can be partially reversed by ICV injection of 5-hydroxytryptophan (5HTP), the precursor of
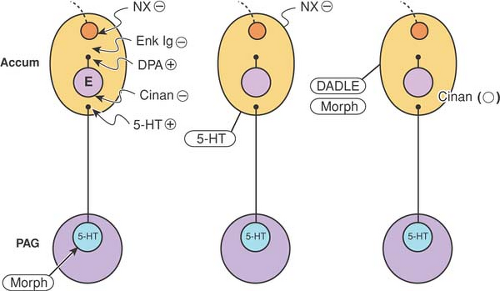
5HT (15). However, no depletion in cerebral 5HT occurred, nor did any decrease in the number of 5HT receptors in the brain of the EA-tolerant animal occur. These negative findings suggest that EA tolerance is likely to be complex and multifactorial. Tolerance to EA analgesia in the rabbit can be reversed by microinjection of 5HT into the nuclei accumbens (16) (Fig. 34-2).
5HT (15). However, no depletion in cerebral 5HT occurred, nor did any decrease in the number of 5HT receptors in the brain of the EA-tolerant animal occur. These negative findings suggest that EA tolerance is likely to be complex and multifactorial. Tolerance to EA analgesia in the rabbit can be reversed by microinjection of 5HT into the nuclei accumbens (16) (Fig. 34-2).
Catecholamines
A series of observations in rat and rabbit suggest that the actions of dopamine and norepinephrine (also called noradrenaline) are antagonistic to AA (17,18,19). Intracerebroventricular injection of apomorphine, a dopamine receptor agonist, reduced the effect of AA, whereas spiroperidol, a dopamine receptor antagonist, increased the effect of AA. Selective destruction of ascending noradrenergic fibers by bilateral microinjection of 6-hydroxydopamine (6-OHDA), the chemical depleter of neuronal noradrenaline, into the medial forebrain bundles in rats reduced the cerebral norepinephrine content and significantly increased the effect of AA. Intraperitoneal injection of norepinephrine, which increases the norepinephrine level in the blood but not in the brain, had no significant effect on AA. This implies that intracerebral norepinephrine antagonizes the effect of AA.
In experiments with rabbits, ICV injection of the α2-adrenergic agonist clonidine antagonized AA, whereas the α1-adrenergic antagonist phentolamine augmented it. Corresponding results were observed after intraperitoneal injection of these two drugs in rats. Rabbits given ICV injection of the β-adrenergic receptor agonist isoproterenol or the β-adrenergic
receptor antagonist propranolol showed no significant change in AA effect.
receptor antagonist propranolol showed no significant change in AA effect.
Opioid Peptides
The discovery of the opioid peptides in 1975, followed by the progressive definition of families of opioid peptides, was a major advance in the understanding of many forms of analgesia. Han and colleagues studied the effect on AA and morphine analgesia in rabbits of ICV microinjection of naloxone into specific brain sites (20). Localization studies determined that the nuclei accumbens, amygdala, habenula, and PAG are particularly important sites at which endogenous opioids exert their analgesic effects. Electroacupuncture analgesia and morphine analgesia involve both spinal and supraspinal mechanisms, with confirmatory evidence for this from spinally transected rats (21).
The endogenous opioid peptide enkephalin is known to be degraded mainly by two enzymes: the dipeptidyl carboxypeptidase enkephalinase, and aminopeptidase. Microinjection of the enkephalinase inhibitor thiorphan or the aminopeptidase inhibitor bestatin into the nucleus accumbens of the rabbit produced a dose-dependent analgesic effect (22). This analgesic effect was completely reversed by naloxone and by antibodies against met-enkephalin administered at the same site. Antibodies against leu-enkephalin were not effective. Moreover, microinjection of thiorphan or bestatin into the nucleus accumbens resulted in a marked potentiation of the after-effect of EA-induced analgesia, as well as analgesia induced by a small dose of morphine. Therefore, the analgesic effect elicited by EA and morphine is mediated, at least in part, by met-enkephalin–like substances in the nucleus accumbens.
Particular attention was focused on the potent analgesic effect of the endogenous analgesic peptide dynorphin injected into the subarachnoid space of the spinal cord of the rat (23,24,25). Site specificity was shown, in that spinal cord (intrathecal) injection of dynorphin A (1,2,3,4,5,6,7,8,9,10,11,12,13) potentiated morphine analgesia whereas brain (ICV) injection antagonized it (26). Intrathecal injection of dynorphin in the rabbit elicited marked analgesia, as measured by TFL, reversible by naloxone, and intrathecal injection of antidynorphin antibody reduced AA by 77%, with the effect lasting for at least 4 hours. No analgesia resulted from dynorphin injection in the PAG, nor was EA analgesia blocked by antidynorphin antibody injected into the PAG. These results suggest that dynorphin reduces nocifensive responses by acting at the spinal cord, and it may play an important role in mediating EA analgesia at the spinal level (27).
Han and colleagues have used highly specific antisera, or the immunoglobulin (Ig)G fractions of such antisera, injected by ICV, intranuclear, or intrathecal routes, to inactivate the target neuropeptides released into the synaptic clefts, leaving the other neuropeptides intact to act on their relevant receptors. The pilot experiments on rats were done “blind”: antisera or their IgG fractions against met-enkephalin, β-endorphin, dynorphin, and substance P (SP), together with
normal rabbit sera, were sent coded from Terenius at Uppsala University to Beijing Medical College. Injections were given intrathecally or into the PAG of the rabbit to assess their effect upon AA. Similar experiments were performed in collaboration with Goldstein at Stanford University, and Herz and Hollt at the Max Planck Institute of Psychiatry in Munich (28,29,30,31,32,33). The results indicate that β-endorphin mediates the effect of AA in the PAG, but not in the spinal cord, dynorphin mediates AA in the spinal cord but not in the PAG, and met-enkephalin contributes to AA through actions both in the brain and in the spinal cord.
normal rabbit sera, were sent coded from Terenius at Uppsala University to Beijing Medical College. Injections were given intrathecally or into the PAG of the rabbit to assess their effect upon AA. Similar experiments were performed in collaboration with Goldstein at Stanford University, and Herz and Hollt at the Max Planck Institute of Psychiatry in Munich (28,29,30,31,32,33). The results indicate that β-endorphin mediates the effect of AA in the PAG, but not in the spinal cord, dynorphin mediates AA in the spinal cord but not in the PAG, and met-enkephalin contributes to AA through actions both in the brain and in the spinal cord.
A later experiment demonstrated involvement of endogenous orphanin FQ (OFQ) in EA analgesia (34). The results suggested that endogenous OFQ exerts a tonic antagonistic effect on EA analgesia, but no such antagonism was observed with intrathecal injection of OFQ. It appeared that spinal OFQ produced a marked analgesic effect and enhanced EA analgesia, consistent with the experimental results in rats, in which morphine analgesia is antagonized by ICV OFQ and potentiated by intrathecal OFQ.
Substance P
Early data in the rat (30), summarized in a review from Han and colleagues (34), indicated that EA releases SP in the PAG, which then accelerates the release of met-enkephalin to produce an antinociceptive effect. In contrast to the actions of SP in the brain, intrathecal injections of SP antiserum augment the effect of AA, consistent with SP’s putative role as a neurotransmitter in primary afferent neurons and candidate for nociceptive transmission at the spinal level. Substance P may therefore be regarded as a neurotransmitter playing opposite roles in different parts of the CNS in mediating the effect of AA.
γ-Aminobutyric Acid
Although γ-aminobutyric acid is an important inhibitory neurotransmitter in the CNS, work to date has addressed only its effect on AA (34,35). First, in the rat, intraperitoneal injection of 3-mercaptopropionic acid (3-MP), which blocks both the synthesis and release of GABA, results in a marked potentiation of the effect of AA. The potentiating effect of 3-MP is completely abolished by prior administration of the GABA degrading enzyme inhibitor amino-oxoacetic acid (AOAA). Second, elevation of cerebral GABA content by AOAA is accompanied by a decrease in the effect of AA. This inhibitory effect of AOAA is reversed by the GABA receptor blocker bicuculline methochloride. Third, ICV injection of another GABA-transaminase inhibitor, γ-vinyl-GABA (GVG), produced a fourfold increase in cerebral GABA content and an associated 80% decrease in the effect of AA 12 to 24 hours after ICV GVG administration. A negative correlation was found between the cerebral GABA content and the effectiveness of AA (r = 0.78, p <0.01).
To locate the sites of action for GABA’s antagonism of AA, compounds affecting GABA metabolism were injected into the PAG of the rat. Intra-PAG injection of 0.4 μmol of 3-MP produced a 193% increase in the effect of AA, whereas 0.5 to 1.0 nmol of the GABAergic agonist muscimol or 0.1 μmol of GVG reduced the EA effect by more than half. These results suggest that the PAG is one of the target areas for GABA to suppress AA. The GABAergic pathway from habenula to dorsal raphe has been implicated for this effect (36).
Spinal cord GABA does not seem to be involved in the mechanisms of AA; for example, no significant effect on AA was found even when a tenfold dose of 3-MP (5 μmol) was injected intrathecally into the rat (Fig. 34-3).
Cholecystokinin Octapeptide
Considerable experimental evidence from the rat suggests that cholecystokinin octapeptide (CCK-8) provides negative feedback that modulates opioid analgesia (37). Evidence supporting this proposed role of CCK-8 in AA includes:
Suppression of opioid analgesia by CCK-8. Rats given EA (15 Hz, 3 V) at specific sites on the hind legs for 10 minutes developed analgesia, as determined by a prolongation of TFL at the end of EA stimulation, an effect that is decreased in a dose-dependent fashion by CCK-8 administered ICV or intrathecally. Antagonism of EA analgesia by CCK-8 was also observed in electrophysiologic and neurochemical studies (38). Electroacupuncture analgesia was shown to inhibit the activity of excitatory nociceptive neurons in the nucleus parafascicularis of the rat and to excite inhibitory nociceptive neurons. These effects of EA were abolished by ICV injection of CCK-8, confirming the results obtained in intact rats that CCK-8 serves as a powerful endogenous modulator antagonizing the antinociceptive effect of EA stimulation (Fig. 34-4).
Potentiation of opioid analgesia by CCK antagonist. Electroacupuncture stimulation in the rat produced a marked increase of CCK-8 immunoreactivity in spinal cord perfusate (39). The increase was greatest in response to EA of 100 Hz and 15 Hz, and less marked in response to 2 Hz. Since CCK-8 has been shown to possess potent antiopioid activity at the spinal level, blockade of the spinal CCK effect would be expected to potentiate EA-induced analgesia, which is known to be opioid mediated. Intrathecal administration of the CCK-B antagonist L-365,260 did not by itself influence TFL but potentiated EA-induced analgesia in a dose- and frequency-dependent manner. This potentiation was most marked at a dose range of 2.5 to 5.0 ng intrathecally, and at a frequency rank order of 100 Hz >15 Hz >2 Hz. The results suggest that an increased release of CCK-8 following EA may limit the effect of opioid peptides, and that the CCK-B receptor mediates the antiopioid effect of CCK-8 in rat spinal cord.
Reversal of opioid tolerance by CCK-8 antiserum. As noted, the analgesic effect of EA in the rat was dose-dependently antagonized by ICV or intrathecal injection of CCK-8 (40). This effect had an immediate onset and lasted for at least 4 hours. CCK-8 alone, however, did not affect baseline TFL. Rats subjected to prolonged EA developed tolerance to EA as well as cross-tolerance to morphine. These tolerances could be postponed or reversed by ICV or intrathecal injection of antiserum against CCK-8. Although CCK-8 antagonized opioid analgesia, it did not affect analgesia induced by 5HT or norepinephrine. Moreover, CCK-8 antiserum did not alter the baseline nociceptive responses, nor did it potentiate EA analgesia in naïve rats. It was concluded that prolonged EA stimulation results in a substantial release of endogenous opioids that in turn evoke the release of CCK-8 in the CNS, dampening the opioid component of EA analgesia. This mechanism may mediate, at least in part, the development of EA tolerance. Research in relation to EA tolerance accompanying repeated needle insertion is not reviewed in this chapter, given its focus upon neural blockade.
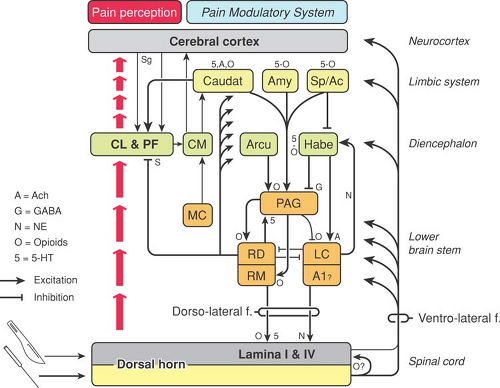
Figure 34-3. Possible mechanisms of acupuncture analgesia. A1, perikarya of noradrenergic neurons with descending fibers to the spinal cord; Ac, nucleus accumbens; Amy, amygdala; Arcu, arcuate nucleus; Caudat, caudate nucleus; CL, controlateralis nucleus of the hypothalamus; CM, nucleus centromedianus of the hypothalamus; Habe, habenular nucleus; LC, locus coeruleus; MC, nucleus megalocellularis; PAG, periaqueductal gray; Pf, nucleus parafascicularis; RD, nucleus raphe dorsalis; RM, nucleus raphe magnus; Sp, septum. From Han JS. Physiologic and neurochemical basis of acupuncture analgesia. In: Cheng TO, ed. The International Textbook of Cardiology. New York: Pergamon, 1986:1124–1132, with permission.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access