Neural Physiology and Local Anesthetic Action
Gary R. Strichartz
Els Pastijn
Kenji Sugimoto
Basic Physiology and Pharmacology
Overview of the Neuron
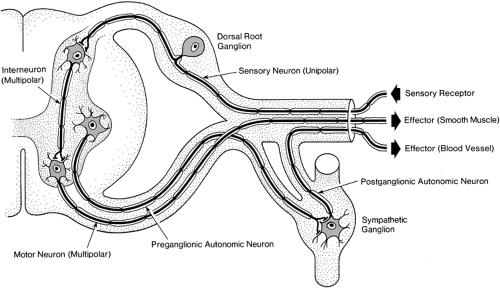
Local anesthetic drugs reversibly block conduction of nerve impulses at the level of the axonal membrane. In this overview, we review briefly the structure and function of neurons, the impulse generating and conducting cells of the nervous system (1). A typical neuron is composed of a cell body (or soma); dendrites, with multiple small branches close to the cell bodies; and a single long axon for each neuron (Fig. 2-1).
Sensory neurons have their cell bodies in dorsal root ganglia. Only one axon is attached, with its longer branch extending to the periphery and a shorter branch to the spinal cord. Impulses are generated in the small peripheral axon branches at the receptor component of the neuron. The distal nerve endings reside in skin, joints, muscles, viscera, or connective tissue. Impulses may be selectively initiated by mild mechanical, thermal (hot or cold changes in skin temperature), or intense tissue-damaging (noxious) stimuli at the nerve endings, whose anatomic spread determines the receptive field for that particular neuron. Intense mechanical and thermal stimuli that can cause pain lead directly to the opening of ion channels selectively responsive to large mechanical distortions or high temperatures. A widespread example of the latter type is the receptor TRPV1, a member of the transient receptor potential (trp) class of channels that exists in many pain fibers. Tissue damage and inflammation also can result in the release of sensitizing chemicals (e.g., bradykinin, prostaglandins) from tissues closely surrounding the endings of nociceptive afferent axons. Such sensitization results in a larger response of nociceptors to specifically noxious stimuli (hyperalgesia) and also to the sensation of pain from stimuli that normally do not cause pain (allodynia). The resulting local depolarization of the nociceptor nerve endings by noxious stimuli leads to trains of impulses with average discharge frequencies that are proportional to the stimulus intensity above the threshold level for impulse generation. Axons then conduct these impulses to the spinal cord, although impulses also invade the soma.
Several axons usually innervate a particular receptive field. As these axons have branches with receptive fields overlapping those of neighboring axons, and each branch alone generates trains of impulses, a convergence occurs in the spinal cord that results in both spatial and temporal summation of afferent impulses. However, the dorsal horn, where primary afferent fibers synapse on second-order neurons, is not merely a relay for transmitting sensory signals. Complex interactions between incoming tactile and nociceptive fibers, as well as modulation by axons descending from the brain, impress sophisticated processing on pain-related activity; in general, these descending pathways have the effect of diminishing pain. As a result, many specifically acting drugs are targeted to receptors for descending axons in the spinal cord to exert a selective analgesia (see Chapters 32 and 33).
Motor neurons have their cell bodies in the ventral horn of the spinal cord gray matter (Fig. 2-1). They are multipolar, in that they have many dendrites in addition to one axon that follows a long course to the periphery. The dendrites and cell body of the motor neuron are specially developed for integrating postsynaptic currents in order to determine the output activity, which occurs as impulse generation. The axon conducts these impulses to its branched, distal terminal enlargements, which contain neurotransmitters to activate effector organs. Skeletal muscles contract rapidly in response to impulses in the large, Aα-fibers that excite them, but their contraction is also strongly dependent on small, myelinated Aγ-fibers that adjust the length threshold for the muscle’s response. We will see later, in the section on Fiber Size and Function, how drug effects on Aγ-fibers can account for much of the motor blockade during peripheral nerve block.
Axons and Peripheral Nerves
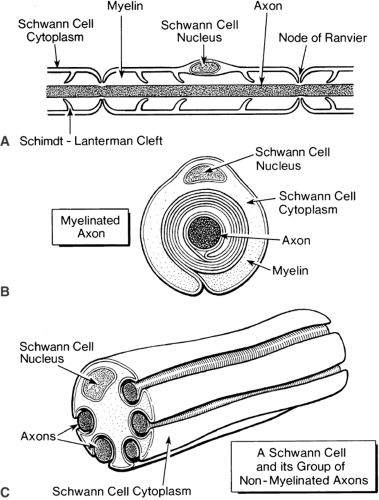
Axons are cylinders of axoplasm encased in the axonal membrane, which is similar to other plasma membranes (Fig. 2-2). Axons are always enveloped by a Schwann cell. Many unmyelinated axons lie within invaginations of a single Schwann cell (Fig. 2-2C), in contrast to the thick myelin sheath of a myelinated axon, which is formed by a single Schwann cell wrapped many times around the axon (Fig. 2-2B). This myelin sheath is interrupted periodically at the nodes of Ranvier, where the extracellular medium has access to the axolemma (Fig. 2-2A).
Nerve impulses travel along nonmyelinated axons as a uniform wave, similar to the way a flame progressively ignites the fuse of a firecracker. The nerve impulse, or action potential, is a change in the electrical voltage across the membrane that is due to changes in the permeability of ionic channels in the axon membrane (2,3). In nonmyelinated axons, these permeability changes occur relatively uniformly along the axon, supporting a wave of inward ion current that underlies the depolarization of the nerve impulse. In myelinated axons, however, changes in the axon’s membrane permeability and the associated inward currents occur only at the nodes of Ranvier; the myelinated internode of the axon is depolarized by the passive spread of current from the nodes (4). Thus, impulse conduction in these axons is continuous but not homogeneous.
Nerve Membranes
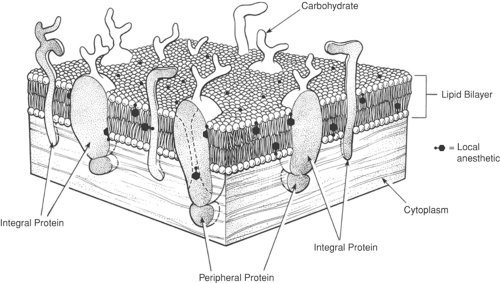
The modern fluid mosaic model depicts the plasma membrane as a bilayer of phospholipid molecules with their fatty acyl chains facing each other, thus making the inner portion of the membrane hydrophobic (Fig. 2-3). The surfaces of the membrane facing the cytoplasm and extracellular fluid are formed by the charged and polar hydrophilic groups of the phospholipid molecules. Globular proteins are also present, and some of these penetrate through the entire thickness of the membrane. Ionic channels are composed of such transmembrane proteins, as are various transporters and receptors for peptide and nonpeptide transmitters and hormones. Such proteins are heavily glycosylated by carbohydrate groups attached covalently to their extracellular surface and often are covalently bound to lipophilic fatty acids that stabilize their transmembrane domains in the hydrocarbon core of the membrane bilayer (5). An intracellular matrix of cytoskeleton is frequently linked to membrane proteins by other specific proteins. All these attachments secure active proteins within the membrane and also can respond dynamically to cellular activity and metabolism to change the protein populations on the cell membrane. As described later (in Basic Pharmacology), local anesthetic molecules are distributed primarily near the interface of membranes, with aqueous solutions that surround them, and also at protein–membrane interfaces and, sometimes, in the ion-conducting pores of membrane channels. Figure 2-3 shows lidocaine’s membrane and protein distribution at the density of drug that occurs for the critical blocking content, which corresponds to one lidocaine molecule for about 100 phospholipid molecules.
Organization of a Peripheral Nerve
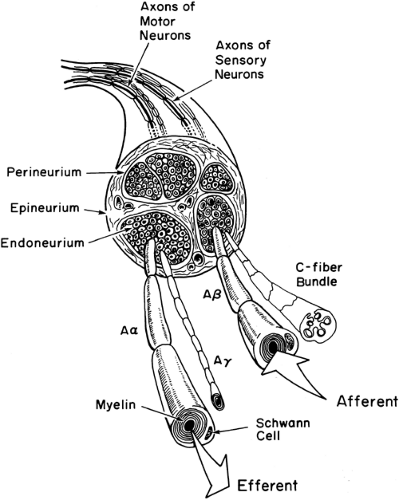
In clinical practice, local anesthetics must diffuse across a number of structures before reaching their site of action in the axonal membrane (6). Peripheral nerves contain both afferent and efferent axons (Figs. 2-1 and 2-4). These individual axons and their Schwann cells are linked loosely together by a delicate layer of fine connective tissue (endoneurium) that allows the easy diffusion of most local anesthetics.
Bundles of axons are enclosed in a squamous epithelial cellular “sheath,” the perineurium, which comprises several layers of cells and acts as a semipermeable barrier to local anesthetics (7,8,9). One or more perineurial bundles are covered by an outermost, easily permeable, connective tissue layer, the epineurium. This layer also carries the nutritional blood vessels of larger nerves. Factors that have an important influence on local anesthetic diffusion to the axons include the thickness of the perineurium, the presence or absence of myelin, the size of the axons, and the anatomic position of the axons, either closer to the outer, more superficial mantle layers of the nerve or deeper within the inner “core” sections of the nerve.
Nerve Membranes and Impulses
The generation and propagation of impulses in excitable nerve and muscle cells depend on the flow of specific ionic currents through channels that span the plasma membrane (2). These channels open and close in response to the electrical potential of the cell membrane and are the targets for local anesthetics as they block impulse propagation. This chapter describes the
role of ion channels in impulse behavior that account for many of the physiologic effects of local anesthetics.
role of ion channels in impulse behavior that account for many of the physiologic effects of local anesthetics.
Most ion channels appear to be very similar in the peripheral nervous system (PNS) and central nervous system (CNS), and in cell bodies as well as axons. Thus, a single description will serve to characterize the essential actions of local anesthetics associated with blockade of impulses anywhere in the nervous system.
Ionic Currents of the Nerve Impulse
Two factors combine to create electric potentials in cells: (a) concentration gradients of ions across membranes and (b) selective permeation of ions through membranes. The gradients are diffusional forces that tend to move the ions; the selective changes in permeability permit that tendency to be manifested as ionic current. Energy from the cell’s metabolism is used to create and maintain the gradients.
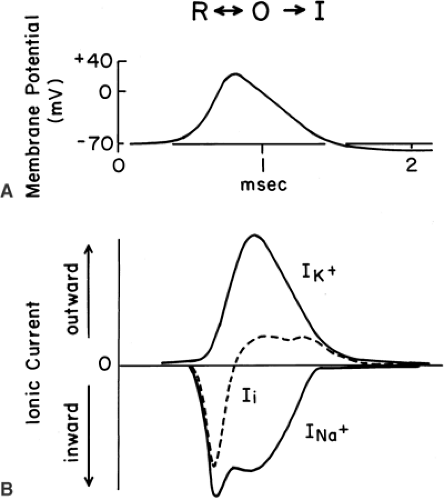
The concentration of potassium ions (K+) inside a cell is about 10 times greater than the extracellular K+ concentration, and vice versa for sodium ions (Na+). A special protein in the membrane (the Na/K pump) actively transports K+ into the cell and Na+ out of the cell, using adenosine triphosphate (ATP) as the source of energy (10). In the resting cell membrane, a selective permeability to K+ ions exists, permitting the net efflux of a small number of K+ ions, thus leaving the axoplasm electrically negative (polarized) while making the outside electrically positive. This accounts, for the most part, for the cell’s “resting potential,” which typically equals -60 to -70 mV (Fig. 2-5).
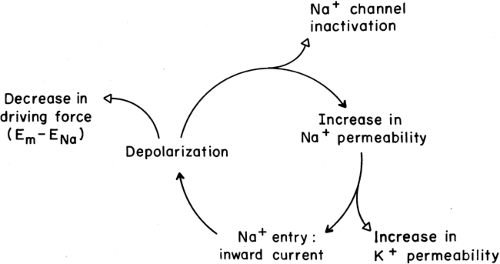
At rest Na+ ions tend to flow into the axon, both because the resting axon is electrically negative inside and because the Na+ ions are more concentrated outside. During a nerve impulse, a selective permeability to Na+ arises when specific Na channels in the axon membrane are opened (2,3). Figure 2-5 shows the contributions of ionic currents during a conducted nerve impulse. The large inward Na current (INa+) accounts for the depolarizing phase of the impulse. Opening of Na+ channels occurs as the membrane is initially depolarized from the resting potential; that is, as the potential becomes less negative. This opening, a result of complex channel activation, is an intrinsic behavior of Na+ channels, due to charged amino acids that are part of the channel molecule’s structure (11). As the membrane depolarizes and the potential becomes less negative, more Na+ channels open, and they open more rapidly. As more channels open, more Na+ ions enter the cell, and the resulting depolarization is accelerated (Fig. 2-6). This positive feedback cycle accounts for the “regenerative” behavior of nerve impulses (2).
Action potentials are brief, transient events. Repolarization of the membrane to the negative resting value occurs because of three factors: (a) The driving force moving Na+ into the cell diminishes as the axoplasmic potential becomes less negative and approaches the Nernst potential for Na+(∼ +50 mV), (b) the sodium channels eventually close (inactivate) during a depolarization, and (c) voltage-dependent K channels open and permit a large, outward K+ current that returns the axoplasmic potential to or beyond, its resting value (Fig. 2-5). In simple terms, impulses have a depolarization phase and a repolarization phase. Inward currents, carried by Na+ ions, depolarize the cell, whereas outward currents, carried by K+ ions, repolarize the cell. The integrated result of all these factors is diagrammed in Figure 2-6, which summarizes the ionic contribution to the nerve impulse.
With each action potential, a pulse of Na+ ions enters the cell and a pulse of K+ leaves it. Small changes in the concentrations of these ions can build up during repeated discharge and thus lessen the ion gradients. However, the Na+/K+-ATPase (Na+/K+ pump), which is activated by elevation of intracellular Na+, will return this Na+ to the extracellular space and, reciprocally, lower the extracellular K+, removing it to the intracellular compartment, using the energy of ATP. This “electrogenic” pump generates an outward current during this process and thereby hyperpolarizes the cell, contributing to an after-potential (10) and shaping the patterns of impulses that occur in constant or intermittent trains of discharge.
Impulse Propagation
Inward current entering the axon during the depolarizing phase of an impulse flows within the conducting medium of the axoplasm and thereby spreads to adjacent, inactive regions. These adjacent regions are depolarized by this “local circuit” current, usually to levels far in excess of those for threshold conditions,
and the regenerative impulse then extends to this region, generating its own inward current. The propagating wave of potential spreads far along axons during any one instant, over distances that are much larger than the fiber’s diameter. For example, if an impulse lasts for 1 msec, from the beginning of its depolarizing phase to the time of initial repolarization, and is conducted at a velocity of 1 m/sec, as in the small, nonmyelinated C fibers (of diameter less than 1 μm), then the action potential extends over 1 mm along the axon, 1,000 times the axon’s diameter. For large myelinated axons, 20 μm in diameter, impulses travel at 60 to 100 m/sec and thus extend over distances of 60 to 100 mm. Inward currents from all the active nodes add together as they spread toward inactive regions, ensuring that impulse propagation will continue. Therefore, complete block of impulses in about three to five nodes in sequence is necessary for the total prevention of impulse propagation in myelinated fibers (4,12,13). Furthermore, the “action current” flowing into the nerve under the impulse is five to ten times that required to depolarize the next segment to threshold; this ratio is referred to as the margin of safety for impulse conduction. Due to this large margin of safety, about 80% of the Na channels in an axon membrane must be inhibited in order to fully block a propagated impulse.
and the regenerative impulse then extends to this region, generating its own inward current. The propagating wave of potential spreads far along axons during any one instant, over distances that are much larger than the fiber’s diameter. For example, if an impulse lasts for 1 msec, from the beginning of its depolarizing phase to the time of initial repolarization, and is conducted at a velocity of 1 m/sec, as in the small, nonmyelinated C fibers (of diameter less than 1 μm), then the action potential extends over 1 mm along the axon, 1,000 times the axon’s diameter. For large myelinated axons, 20 μm in diameter, impulses travel at 60 to 100 m/sec and thus extend over distances of 60 to 100 mm. Inward currents from all the active nodes add together as they spread toward inactive regions, ensuring that impulse propagation will continue. Therefore, complete block of impulses in about three to five nodes in sequence is necessary for the total prevention of impulse propagation in myelinated fibers (4,12,13). Furthermore, the “action current” flowing into the nerve under the impulse is five to ten times that required to depolarize the next segment to threshold; this ratio is referred to as the margin of safety for impulse conduction. Due to this large margin of safety, about 80% of the Na channels in an axon membrane must be inhibited in order to fully block a propagated impulse.
In the face of reduced membrane excitability due to disease, altered metabolism, or drug action, impulses may still occur but will be conducted more slowly. When the net inward current is decreased, the action potential amplitude will be smaller, the rate of impulse depolarization slower, and the velocity of conduction lower. This condition often worsens as an impulse attempts to propagate through a diseased or drugged axon, a situation termed decremental impulse conduction. If the decrement is sufficiently great, conduction failure occurs; if not, then only conduction slowing along the affected axonal region occurs, and normal impulse conduction resumes if the decremented action potential is able to excite the contiguous normal region of the axon. This situation certainly occurs for nerve conduction during the onset and regression of a local anesthetic block. Even in an effectively, fully blocked axon, conduction fails decrementally as an impulse enters the anesthetic-exposed region of the nerve rather than stopping abruptly in a very short distance.
Mechanisms of Anesthetic Action
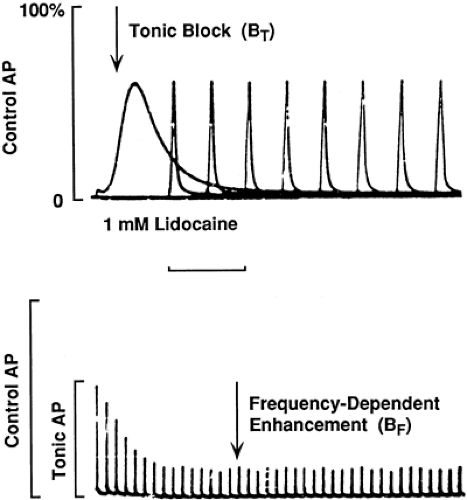
Local anesthetics block impulses by interfering with the function of Na channels (14,15). In the presence of local anesthetics, Na channels are less likely to open in response to a stimulating depolarization (16,17,18), the resulting Na+ current is decreased, and, at sufficiently high anesthetic concentrations, enough channels are impaired to prevent impulse generation. When a local anesthetic–containing solution is applied to a desheathed peripheral nerve in vitro, inhibition is detected in a minute and a steady-state level of block is usually achieved in 10 minutes or less. At low frequencies of impulse firing, 1 Hz (1/sec) or less, this “tonic block” has a constant value (Fig. 2-7A). Higher-frequency impulses increase the degree of block, a phenomenon called phasic or use-dependent block (22), which quickly reaches a new steady state (Fig. 2-7B) and quickly reverses to the tonic block level when the stimulation frequency returns to a very low level.
These changes in impulse behavior are caused by reciprocal drug-induced changes in the opening and closing of Na+ channels. Both tonic and phasic block can be accounted for by
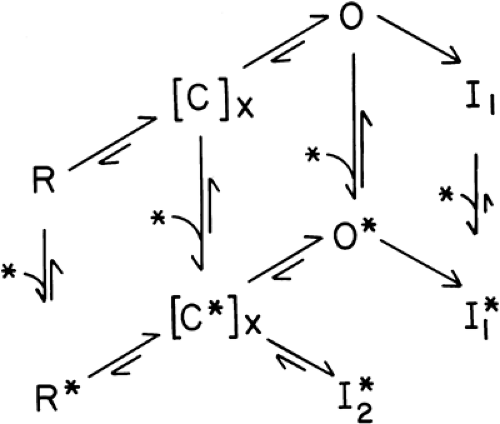
the modulated receptor hypothesis (MRH) (19,20) (Fig. 2-8). This hypothesis rests on the accepted notion that Na+ channels normally respond to membrane depolarizations by passing through defined conformational “states,” beginning at rest (R), activating through closed intermediate forms (C) to reach an open (O) form—the “activation” response—and then closing to an inactivated (I) state. According to the MRH, local anesthetics have a higher affinity for open and, especially, inactivated Na+ channels than they do for resting channels (21,22). During stimulation, some of the channels that are opened and then become inactivated bind local anesthetics more tightly than the resting channels did. This additional binding thus stabilizes the channels in a nonconducting state, and the fraction of channels so bound increases with each stimulating pulse.
Some anesthetic-bound channels will return to the resting, unbound state, but much more slowly than inactivated, drug-free channels recover, so that eventually a steady-state level of phasic block will be reached wherein increased drug binding during a depolarization is exactly reversed by drug dissociation in the time between pulses.
the modulated receptor hypothesis (MRH) (19,20) (Fig. 2-8). This hypothesis rests on the accepted notion that Na+ channels normally respond to membrane depolarizations by passing through defined conformational “states,” beginning at rest (R), activating through closed intermediate forms (C) to reach an open (O) form—the “activation” response—and then closing to an inactivated (I) state. According to the MRH, local anesthetics have a higher affinity for open and, especially, inactivated Na+ channels than they do for resting channels (21,22). During stimulation, some of the channels that are opened and then become inactivated bind local anesthetics more tightly than the resting channels did. This additional binding thus stabilizes the channels in a nonconducting state, and the fraction of channels so bound increases with each stimulating pulse.
Some anesthetic-bound channels will return to the resting, unbound state, but much more slowly than inactivated, drug-free channels recover, so that eventually a steady-state level of phasic block will be reached wherein increased drug binding during a depolarization is exactly reversed by drug dissociation in the time between pulses.
The normal inactivated state is not essential for tonic or use-dependent block (23,24). It appears that both open and activated channels react most rapidly with anesthetic molecules and that inactivated channels react more slowly but still have a greater equilibrium drug affinity than resting channels (21). (Clinically, during one, relatively brief nerve impulse [0.5–1 msec], the presence of inactivated channels is limited and drug binding to this state probably contributes little to the observed inhibition. In contrast, cardiotoxicity will arise from this slow binding to cardiac Na+ channels that remain inactivated during the 200 msec of the action potential’s plateau) (25,26). The concept of selective, state-dependent binding of anesthetics lies at the core of the MRH.
Since they lessen the probability that channels will open, local anesthetics have an effect like that of slow depolarization. They shift channels toward an “inactivated-like” state that cannot be directly opened by stimulation (16,19,20). The major difference is that recovery from normal, depolarization-dependent inactivation is fast (a few milliseconds), whereas recovery that depends on local anesthetic unbinding is slow (0.1–1 seconds). In this way, local anesthetics dramatically increase the effective refractory period and thus limit the frequency with which a nerve can fire.
Basic Pharmacology
Exploration of the mechanism of local anesthetic action raises three fundamental questions: Which species of the drug, neutral or protonated, is the active form? Where does the anesthetic molecule act? What is the molecular mechanism of Na+ channel interference?
Active Species: The Role of Ionization
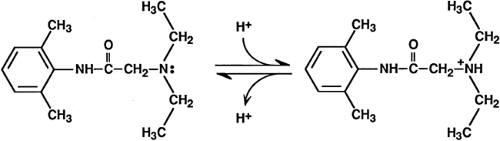
Under physiologic conditions, most local anesthetics exist in rapid equilibrium between the neutral and protonated, charged species (Fig. 2-9). The ratio of ionized (LH+) to nonionized (L) molecules is given by the Henderson-Hasselbalch equation:
where the pKa is the value of pH at which the concentrations of the unionized and ionized species are equal. The pKa values of clinically used local anesthetics range from about 7.7 to 9.5 (27), so that in solution all these drugs are charged more often than they are uncharged. Protonation and deprotonation in solution are very rapid processes, occurring about 103 times per second. When the drug is buried in a membrane or bound to a protein molecule, however, these reactions can be much slower.
Both ionized and nonionized drug species can inhibit Na+ channels. Quaternary derivatives of local anesthetics, which are permanently charged, when placed inside a neuron are potent blockers of impulses and of Na+ currents (28,29). Interestingly, these drugs have very slowly developing effects when present outside of the axon; their constant charge and low hydrophobicity greatly restrict their passage into and through the membrane.
Perpetually uncharged local anesthetics also effectively block impulses and Na+ channels (30,31,32), demonstrating that ionization is not essential for pharmacologic activity. The ability of tertiary amine local anesthetics to block Na+ channels in single cells is faster and greater when the drug is applied externally at alkaline external pH (33,34,35), or when it is applied by direct internal perfusion at neutral or slightly acidic internal pH (36). Both of these observations are consistent with a model in which the neutral form of the anesthetic dissolves in and passes through the axon membrane and, having reached a cytoplasmic region, becomes protonated.
Changes in the binding and dissociation of local anesthetics by direct mutation of certain amino acids of the large, α-subunit of the channel demonstrate that the drug binds in the inner “pore” of the Na channel (37,38,39,40) (Fig. 2-10). One intriguing finding is that dissociation of anesthetic molecules from the closed channel is clearly sensitive to the pH outside the nerve, but relatively insensitive to the intracellular pH (34). Thus, when it is bound in the Na+ channel, a local anesthetic molecule can interact with extracellular protons. Since the changes in channel structure that underlie its gating also contribute to changes in local anesthetic affinity (see the earlier section, Mechanisms of Anesthetic Action),
other factors that influence gating can modify drug blockade. Among these are the concentration of calcium (Ca2+) ions outside the cell (19) and the identity of the smaller β-subunits that are associated with the large α-subunit (41).
other factors that influence gating can modify drug blockade. Among these are the concentration of calcium (Ca2+) ions outside the cell (19) and the identity of the smaller β-subunits that are associated with the large α-subunit (41).
Hydrophobicity and Potency
Drug hydrophobicity is also a determinant of potency. The more hydrophobic drugs are more potent blockers of action potentials (42,43) and of Na currents (44,45,46). This increased potency results from a faster apparent association rate, as well as from a slower dissociation rate, for the binding of the local anesthetics to their channel sites (21,44). In other words, the more hydrophobic local anesthetics can reach the site more easily and appear to leave the site more slowly than the less hydrophobic drugs. Molecular mass of the anesthetics, however, also affects the dissociation of the charged local anesthetics, but not of the neutral ones (45). These results support the concept of two pathways to and from the anesthetic site (19). One pathway may be through the channel’s pore (the hydrophilic route, more favorable to charged molecules) and the other may be through the membrane (the hydrophobic route, accessible primarily to uncharged drug molecules). Whether these separate routes eventually lead to a single or several sites of action is unclear at this time.
From this discussion, it appears that pH affects anesthetic potency on isolated neurons by two opposing actions. An alkaline extracellular pH favors the neutral, membrane-permeant form of the drug, facilitating the rise of drug concentration in the membrane and thus inside the cell, whereas an acidic extracellular pH favors the more potent protonated blocking species when drug is present at the site of action. Both neutral and charged forms of anesthetics participate in impulse blockade. Of course, during most clinical procedures, the functional potency of local anesthetics is determined largely by the fraction of injected drug that actually passes into the nerve bundle, and conditions that favor the penetrating, neutral species are desirable (6,47). Other local anesthetics probably have about the same unfavorable distribution properties.
Molecular Mechanisms of Sodium Channel Blockade
Exactly how do local anesthetics interfere with the operation of Na+ channels? Such questions are addressed by a combination of physiologic and structural studies. Much of this study is anchored on the observation that anesthetics inhibit open and inactivated channels more potently than resting closed channels, and that intentional mutation of specific amino acid residues believed to line the inner region of the pore (37,39) (along the transmembrane helices labelled S6 in Fig. 2-10), leads to changes in the affinities of local anesthetics for different states of the channel (48,49,50).
Does a local anesthetic molecule inhibit the channel by “plugging” the pore and preventing ions from passing, or by interfering with the conformational changes that underlie channel opening? Both possibilities may occur during the blockade of impulses by tertiary amine drugs, because the channel regions that bind the drug are coupled to those regions that sense the changes in membrane potential and that initially move in order to activate the channel. Other substances that pass through the channel as well as binding within it, such as hydrogen (H+) and certain cations, also change the gating, providing precedents for the coupled interaction between the pore-forming and the gating domains. Some local anesthetics interfere with the conformational restriction of gating regions (i.e., the molecular equivalent of inactivation), inducing an “inactivation-like” state by a drug-dependent mechanism rather than stabilizing the normal conformational changes that occur during inactivation.
Susceptibility of Sodium Channels to Local Anesthetics
A differential susceptibility to a local anesthetic of certain Na+ channel subtypes, for example, those that are selectively expressed in nociceptors more than in motor neurons, would enable a selective analgesia without paralysis, one of the yet-to-be-attained goals for regional anesthesia. Is there experimental evidence to support such channel selectivity?
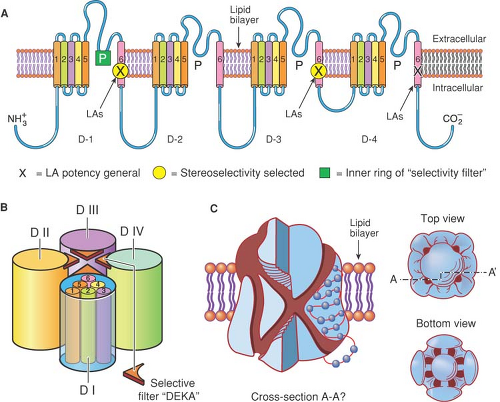
Voltage-gated Na+ channels are composed of one large α-subunit and one or two β-subunits. The α-subunit, of which nine have been functionally identified, conducts all the essential activity on Na+ channels; it contains the “voltage sensor” that moves in response to depolarization, the “ion selectivity filter” that permits the selective permeation of Na+, and the “gating” structures that respond to voltage sensor movement by opening or closing the channel (Fig. 2-10). β-subunits, four identified so far, are covalently or noncovalently attached to the α-subunits, have immunoglobin-like structures, and chaperone the larger subunits to the membrane, anchoring them to cytoskeletal and extracellular structures. β-subunits also modify the gating of Na+ channels, which is an important determinant of local anesthetic binding. The various α-subunits are differentially distributed among different tissues and cell types. For example, the Nav1.8 α-subunit occurs only in certain nociceptor neurons, and Nav1.5 is the major channel protein in cardiac muscle (see Chapter 33).
Selective expression is sometimes changed after tissue injury or inflammation (51,52). For example, Nav1.8 in small diameter dorsal root ganglia (DRG) neurons increases after inflammation but decreases after nerve injury. Expression of the Nav1.3 α-subunit is very low in intact adult DRG but is elevated after nerve injury or inflammation (53) and contributes importantly to late Na+ currents in nociceptors (54,55). β-subunits are also changed by nerve injury, with protein levels of β2 increasing in both injured and neighboring, noninjured sensory neurons (56). Since β-subunits alter both the density of functional Na+ channels as well as their gating properties, they would be expected to contribute to local anesthetic action. Indeed, evidence exists of differential effects on local anesthetic blockade by different β-subunits (41,57,58), some of which may arise from altered gating of the α-subunit, whereas others might depend on direct interactions of part of the β-subunit with the local anesthetic binding site (59).
The differential distribution of Na+ channel subtypes provides the possibility that subtype-selective drugs may modify the target tissue function with fewer side effects on other tissues. In this context, a selective action on pain might be expected from a drug that targets those Na+ channels whose expression is confined to small-diameter sensory afferents (e.g., Nav1.8 or Nav1.9) (44,60,61). Indeed, the limited clinical use of available Na+ channel blocking drugs is due to their lack of specificity for neuronal Na+ channel subtypes (e.g., in comparison to cardiac Na+ channel subtypes). The overall potency of local anesthetics for the Na+ channels is relatively low (19,21,22), and the accompanying stereoselectivity for chiral drugs is weak, rarely exceeding 5 (62,63,64).
Comparison of the amino acid residues believed to bind the Na+ channels in their different states shows little difference among the known channels, consistent with the similar
blocking affinities. Although the amino acid sequence of the α-subunit in homologous domains is quite similar among the different subtypes, particularly for the S6 transmembrane segments that form the cytoplasmic opening of the channel (Fig. 2-10), there may be subtle differences in their spatial orientation in different conformational states of the channel. Furthermore, the contribution of specific residues to local anesthetic binding affinity among the different channel states depends on the local anesthetic molecule, even varying between stereoisomers, for example, R– and S-bupivacaine (64), such that channel structures that can influence S6 positions (e.g., those on S5), or even cytoplasmic or extracellular connecting peptides, may be able to allosterically alter local anesthetic affinities. Changes in the structure of the selectivity filter, closer to the extracellular pore opening, also modify local anesthetic binding affinity and access to the site, implying that ion flow through the channel also regulates local anesthetic binding (16,65,66).
blocking affinities. Although the amino acid sequence of the α-subunit in homologous domains is quite similar among the different subtypes, particularly for the S6 transmembrane segments that form the cytoplasmic opening of the channel (Fig. 2-10), there may be subtle differences in their spatial orientation in different conformational states of the channel. Furthermore, the contribution of specific residues to local anesthetic binding affinity among the different channel states depends on the local anesthetic molecule, even varying between stereoisomers, for example, R– and S-bupivacaine (64), such that channel structures that can influence S6 positions (e.g., those on S5), or even cytoplasmic or extracellular connecting peptides, may be able to allosterically alter local anesthetic affinities. Changes in the structure of the selectivity filter, closer to the extracellular pore opening, also modify local anesthetic binding affinity and access to the site, implying that ion flow through the channel also regulates local anesthetic binding (16,65,66).
Importantly, since state-dependent affinities are a strong feature of local anesthetic inhibition, channels that have different gating behaviors may be blocked differently under the same conditions. For example, the mammalian cardiac Na+ currents, carried by channel Nav1.5, were reported to be more susceptible to lidocaine than are the currents in skeletal muscle (carried by Nav1.4), based on voltage-clamp measurements using the same patterns of membrane potential. Further examination, however, showed that the cardiac channels were more inactivated at the same resting potential, and the apparently higher affinity was actually due to the preferred binding to inactivated channels (67).
When comparing the effect of local anesthetics among different Na+ channel subtypes in sensory neurons, previous reports suggest that local anesthetics are more potent on tetrodotoxin (TTX)-sensitive Na+ current (e.g., carried by Nav1.7) than on TTX-resistant current (carried by Nav1.8, Nav1.9) (68). However, the TTX-sensitive Na+ channels tend to be more inactivated at resting potentials than are the TTX-resistant Na+ channels (69,70). Thus, the difference in local anesthetic sensitivity between TTX-sensitive and TTX-resistant Na+ channels might again be defined primarily by gating differences and should be interpreted with caution.
Ropivacaine’s actions may present an exception to this concern. This local anesthetic is reported to provide clinically relevant differential block, producing analgesia with less intense motor block (71). Unlike other local anesthetics, ropivacaine is more potent in blocking TTX-resistant Na+ currents, such as occur in nociceptors, than TTX-sensitive Na+ channel in DRGs, when the resting membrane potential is set at –80 mV, close to the physiologic condition and at which some of the channels will be in the inactivated state (72). Thus, factors other than gating must account for ropivacaine’s preferential inhibition of the TTX-resistant subtype of channel that occurs in nociceptors.
Direct pharmacologic assays for local anesthetics on Na+ channels determine the potency at the primary target site. However, because the intensity of pain is encoded by the frequency of action potentials in a train of impulses, it is important to note that the local anesthetic concentration for half-maximal blockade of ion currents does not predict the relevant concentration required for a functional blockade of neuronal signal transmission (61). Sensitivity to local anesthetic was higher for reducing the firing frequency than for reducing the peak amplitude of a single TTX-resistant action potential. Thus, an effective dose for pain management might be much lower than the dose for blockade of ion currents. For differential functional blockade, it is essential to realize that the opening and inactivation kinetics of the same subtype of Na+ channel may vary substantially among different cell types that have differently shaped action potentials. For example, local anesthetics might selectively block impulses in one type of cell, which has long-lasting action potentials that allow time for the blockade of prolonged open and inactivated states, while sparing those in another cell type that has very brief action potentials. Since excitability properties such as the duration of action potentials, postimpulse de- or hyperpolarization, and even the resting potential are highly sensitive to K+ channels, which also vary widely in their tissue distribution (73,74), a selective blockade of impulses in one cell type could result from its K+ channel expression profile, independently of any differences in Na+ channel expression.
Finally, Na+ channel gating is modified by enzymes, including protein kinases (75,76), that are activated or suppressed by pathways ordained by G protein-coupled receptors (GPCRs), and/or are influenced by changes in intracellular [Ca2+] influenced strongly by flux through Ca channels. Because local anesthetics also act to inhibit both GPCRs and Ca2+ channels (73,77,78,79,80,81), they may thus have an indirect as well as a direct effect on voltage-gated Na+ channels and cellular excitability.
Clinical Applications for Peripheral and Regional Nerve Blocks
Peripheral Nerve Anatomy and Function
Both anatomical and chemical factors determine the susceptibility of nerve fibers to block by local anesthetics. The particular nerve being blocked and the nature of the agent used and its formulation are important for the rate of onset, maximum degree and duration of nerve block, and differential blockade of different functions by local anesthetics.
Fiber Size and Function
The diameter and myelination of a nerve fiber are correlated with its impulse physiology as well as its message-carrying function (82,83). Nerve fibers are categorized into three major anatomic classes (Table 2-1): myelinated somatic nerves (A-fibers), myelinated preganglionic autonomic nerves (B-fibers), and nonmyelinated axons (C-fibers). The B- and C-fibers are small, ranging in diameter from about 2 μm to less than 1 μm, respectively, whereas the A-fibers vary in diameter from about 4 to 20 μm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree