Neural Blockade: Impact on Outcome
Christopher L. Wu
Spencer S. Liu
The importance of effective management of acute pain is clear from multiple studies documenting the undertreatment of pain and introducing guidelines and regulatory standards for the assessment and treatment of acute pain. The presence of uncontrolled acute pain may potentially result in widespread adverse responses that may contribute to a number of perioperative complications. As described in the preceding chapter by Drs. Schricker and Carli, the effective management of acute pain may attenuate several of these adverse responses and, in turn, improve patient outcomes. Many different classes of analgesic agents and different analgesic techniques exist, each of which has a unique profile (e.g., quality of analgesia, presence of side effects, attenuation of adverse physiologic responses, and impact on outcomes). However, regional anesthesia and analgesic techniques, including neuraxial and peripheral regional analgesia, are especially effective in the provision of postoperative analgesia and attenuation of perioperative pathophysiology. In this chapter, we review the evidence, focusing on available systematic review and meta-analyses, for the effects of regional anesthetic and analgesia techniques (both peripheral and neuraxial) on patient outcomes.
Pathophysiology of Acute Pain
The noxious stimuli from iatrogenic surgical injury or accidental trauma set a cascade of events into motion, cumulating in the perception of “pain.” Many interrelated components contribute to the processing of nociceptive stimuli. These pathways are generally divided into peripheral and central (i.e., spinal and supratentorial) processes. Clinicians should recognize that the neurobiology of nociception is extremely complex, with multiple levels of redundancy such that no “hard-wired” or “final common” pathway exists for the process of nociception. Comprehensive discussions of the neuroanatomy and pharmacology of nociception are provided in Chapters 31,32,33. For the purposes of the present chapter, we will concisely survey these processes and limit our description of central nociceptive processing to that taking place in the spinal cord.
Peripheral Nociceptive Processing
Surgical incision initiates a variety of noxious stimuli (mechanical or chemical) that ultimately are processed, conveyed to higher brain centers, and perceived as “pain.” In the periphery, information from the noxious stimulus is processed by receptors and neurons whose primary function is the processing of nociceptive information. Primary afferent nociceptors, which are distinct from those that carry innocuous somatic sensory information, convert a wide range of environmental noxious stimuli into electrochemical signals. Activation of primary afferent nociceptors may also initiate the process of neurogenic inflammation and the release of neurotransmitters, particularly substance P and calcitonin gene-related peptide (CGRP), that may cause vasodilation and plasma extravasation (1). Release of these neurotransmitters, along with inflammatory mediators (e.g., bradykinin, prostaglandins, serotonin, neurotrophins) released at the site of injury, may initiate the process of peripheral sensitization (1). The electrochemical signals processed by the primary afferent nociceptors are transmitted toward the spinal cord in the small diameter Aδ- and C-fibers that primarily transmit nociceptive information.
Primary sensory afferent nociceptors synapse with neurons in the dorsal horn of the spinal cord. Neurotransmission across the synapse, originating from peripheral afferent Aδ- and C-fibers, is mediated by a number of peptides and amino acids that interact with specific receptors postsynaptically. For example, substance P, which is specific to the small-diameter primary afferents and released by noxious thermal, mechanical, and chemical stimuli in the periphery, will interact with the neurokinin (NK)-1 receptor postsynaptically.
In addition, the excitatory amino acid glutamate is also present within small-diameter primary afferents, released by noxious stimulation, and may interact with one of three receptor classes: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate, N-methyl-D-aspartate (NMDA), and metabotropic glutamate receptors (mGluR). Although postsynaptic interaction occurs with the AMPA, NK-1, mGluR, and NMDA receptors, as described in Chapters 31,32,33, presynaptic interactions may also exist (e.g., mGluR, voltage-gated calcium channels) that may affect neurotransmitter release (2).
Central (Spinal) Nociceptive Processing and Descending Modulation
Although the primary afferents release neurotransmitters to activate the second-order neurons in the spinal cord, the specific actions depend on the particular receptor activated, since the second-order neurons in the dorsal horn contain a wide variety of neurotransmitter receptors. Activation of certain receptors, such as the NMDA, AMPA/kainate, mGluR, and NK-1, results
in depolarization and increased nociceptive pain transmission. Activation of other receptors, such as the opioid γ-aminobutyric acid-A (GABAA) and serotonin, results in hyperpolarization and a decrease or inhibition of nociceptive pain transmission. Actual transmission of nociceptive information may also be modified by descending inhibitory systems including serotonin, enkephalin, and noradrenergic neurons (see Chapters 31 and 32).
in depolarization and increased nociceptive pain transmission. Activation of other receptors, such as the opioid γ-aminobutyric acid-A (GABAA) and serotonin, results in hyperpolarization and a decrease or inhibition of nociceptive pain transmission. Actual transmission of nociceptive information may also be modified by descending inhibitory systems including serotonin, enkephalin, and noradrenergic neurons (see Chapters 31 and 32).
Although not all nociceptive input results in a pathologic process, a certain percentage of patients who undergo surgical procedures will exhibit prolonged central sensitization and chronic pain. Pathologic nociceptive input may cause a central sensitization that is marked by hyperexcitable spinal neurons that exhibit a decreased threshold for activation, increased and prolonged response to noxious input, expansion of receptive fields, possible spontaneous activity, and activation by normally non-noxious stimuli (33). Induction and maintenance of central sensitization emphasizes different receptor–neurotransmitter combinations, including NMDA receptors, prostaglandins, and neuropeptides (substance P, CGRP, neurokinin A) (3). Ultimately, transcriptional changes (including induction of genes), structural changes in synaptic connections (e.g., contact between low-threshold afferent and nociceptive neurons), and loss of inhibitory interneurons may result in a persistent state of central sensitization (4).
Effects on Individual Organ Systems
The noxious stimuli and resultant pathophysiology associated with surgery may affect many organ systems and result in postoperative complications, particularly in patients in certain subgroups (e.g., older patients, those with decreased physiologic reserve, and those undergoing specific procedures such as lung resection or coronary artery bypass [CAB]).
Cardiovascular
It has traditionally been thought that an imbalance of myocardial oxygen supply and demand, such as an increase in demand (e.g., increase in heart rate or blood pressure) or decrease in supply (e.g., decreased coronary blood flow to the vulnerable subendocardial areas), may contribute to perioperative cardiac events particularly in patients with decreased cardiac reserve (5). Although many factors may contribute to an imbalance of myocardial oxygen supply and demand, uncontrolled postoperative pain may be especially detrimental and contribute to cardiac morbidity through activation of the sympathetic nervous system, other surgical stress responses, and the coagulation cascade. Increased sympathetic nervous system activity can increase myocardial oxygen demand by increasing heart rate, blood pressure, and contractility or even decrease myocardial oxygen supply, which in turn may lead to angina, dysrhythmias, and areas of myocardial infarction (5). In addition, sympathetic activation may enhance perioperative hypercoagulability, which may contribute to perioperative coronary thrombosis or vasospasm, thus reducing myocardial oxygen supply (7,8).
Pulmonary
The pathophysiology of pulmonary dysfunction after surgery is multifactorial. Relevant factors include disruption of normal respiratory muscle activity that may result from either surgery or anesthesia, reflex inhibition of phrenic nerve activity with subsequent decrease in diaphragmatic function, and uncontrolled postoperative pain, which may contribute to voluntary inhibition of respiratory activity or splinting (9). Although the pathophysiology of breathing and respiratory muscle function following surgery is complex, it is clear that anesthetic or analgesic agents administered in the perioperative period affect the central regulation of breathing and activities of respiratory muscles. This incoordination of respiratory muscle function (which may last well into the postoperative period) will impair lung mechanics (9), increasing the risk of hypoventilation, atelectasis, and pneumonia. Visceral stimulation may decrease phrenic motoneuron output, which results in a decrease in diaphragmatic descent and lung volumes (9).
Gastrointestinal
Although decreased gastrointestinal (GI) motility is expected after abdominal surgery, return of GI function usually occurs within several days postoperatively. However, some patients will develop paralytic ileus, a protracted and more severe state of GI immotility. Although the pathophysiology of postoperative ileus and decreased GI motility is multifactorial, the primary mechanisms include neurogenic (spinal, supraspinal adrenergic pathways), inflammatory (i.e., local inflammatory responses initiate neurogenic inhibitory pathways), and iatrogenic pharmacologic (e.g., opioids) mechanisms (10). In the acute postoperative phase, neurogenic (spinal and supraspinal) mechanisms are primary mediators of decreased GI motility (10). Activation of splanchnic afferents and increased sympathetic outflow, along with the possible use of opioids, are the predominant mechanisms for decreased GI motility immediately following surgery (10). However, over the subsequent postoperative days, a prolonged phase of postoperative ileus occurs. The presumed etiology of the latter is distinct, and involves an enteric molecular inflammatory response that impairs local neuromuscular function and activates neurogenic inhibitory pathways (10). Our understanding of the mechanisms of postoperative ileus is not complete, and it is likely that these three mechanisms are not discrete phenomena but interrelated (10).
Coagulation
It is recognized that hypercoagulability occurs in association with surgical procedures. Although the pathophysiology of coagulation-related events (e.g., formation of deep venous thrombosis [DVT]) has essentially been unchanged since Virchow’s initial description of the triad of stasis, blood vessel injury, and hypercoagulability (11), our current understanding of the coagulation system is that it is a complex system with many other functions including tissue repair, autoimmune regulation, arteriosclerosis, and tumor growth and metastasis (12). Nevertheless, the primary components of the coagulation systems comprise cellular (e.g., platelets, endothelial cells, monocytes, and erythrocytes) and molecular (e.g., coagulation factors and inhibitors, fibrinolysis factors and inhibitors, adhesive and intercellular proteins, acute-phase proteins, immunoglobulins, phospholipids, prostaglandins, and cytokines) components (12). The normal process of coagulation involves several steps including initiation (damaged vascular endothelium expresses tissue factor, which ultimately leads to generation of thrombin), amplification (augmentation of the effects of thrombin), propagation (formation of clot), and stabilization (formation of a stable fibrin meshwork that protects the clot from fibrinolytic attack) (12). However, following surgery, the normal process of coagulation may become unbalanced, which may result in a tendency toward thrombosis. Immediately after surgical incision, there are increases in levels of tissue factor, tissue plasminogen activator, plasminogen activator
inhibitor-1, and von Willebrand factor, which contribute to a hypercoagulable and hypofibrinolytic state postoperatively (12).
inhibitor-1, and von Willebrand factor, which contribute to a hypercoagulable and hypofibrinolytic state postoperatively (12).
Cognitive Function
The etiology of postoperative cognitive dysfunction (PCD) is uncertain but most likely involves the combination of many factors, including dysregulation of cerebral neurotransmitters, patient factors (e.g., age, comorbidities, preoperative cognitive function and general health), surgical procedure undertaken (certain procedures such as CAB may have a higher incidence), and perioperative drug therapy. It is believed that a perioperative imbalance of neurotransmitter systems, especially acetylcholine and serotonin, or increase in inflammatory mediators, such as cytokines, may contribute to the development of PCD, especially in the elderly, who may have a decreased neurophysiologic reserve at baseline (13,14). Central cholinergic deficiency through anticholinergic mechanisms or impaired acetylcholine production may be an important factor in the development of PCD, with a possible dose-response relationship between the degree of pharmacologic anticholinergic activity and severity of delirium (15). In addition, an excessive amount or enhanced transmission of serotonin, which is important for mediating mood, sleep, and cognition, can result in confusion and restlessness (15). Surgery will result in the release of cytokines, which may ultimately influence neurotransmitter activity and contribute to PCD, since administration of interleukin (IL)-2 has been associated with cognitive dysfunction and delirium (14). Finally, some surgical procedures may be specific etiologies of PCD, especially in patients undergoing cardiac surgery. Besides the possible etiologies of PCD discussed earlier, PCD after cardiac surgery may reflect additional factors including cerebral microembolization, global cerebral hypoperfusion, cerebral temperature perturbations, cerebral edema, and possible blood–brain barrier dysfunction (16).
Immunologic Function
It is clear that patients experience an alteration in immune status following surgery. Following major surgical procedures, an early hyperinflammatory response occurs, with release of proinflammatory tumor necrosis factor (TNF)-α, IL-1 and IL-6 cytokine, neutrophil activation and microvascular adherence, and uncontrolled polymorphonuclear (PMN) and macrophage oxidative activity (17). This hyperinflammatory response is followed by significant cell-mediated immunosuppression marked by monocyte deactivation, decreased microbicidal activity of phagocytes, and an overall imbalance between proinflammatory and anti-inflammatory cytokines and immunocompetent cells (17,18). Surgery in the abdominal cavity may also elicit an enhanced immunologic response, as manipulation of the GI tract initiates an inflammatory cascade within the intestinal wall muscularis that results in a decrease in GI motility (18). Cytokines released into the peritoneal fluid during abdominal surgery may decrease organ function and increase the risk of anastomotic leakage, particularly during sepsis (18). The resultant immunosuppression may lead to an increased risk of postoperative infection and could in theory influence outcomes in oncologic patients. However, it is possible that laparoscopic surgery, which results in less trauma than conventional open surgery, might be associated with a reduced inflammatory response and subsequent immunosuppression due to a decrease in the production of cytokines and in activation of cellular and humoral immune responses (18).
Effects and Efficacy of Acute Pain Management on Postoperative Outcome
Epidural and Spinal Neural Blockade
Mortality
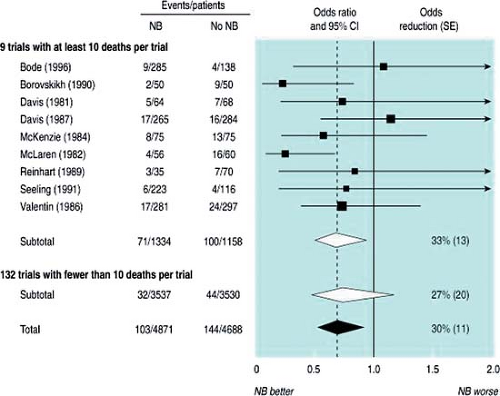
Despite controversy (due in part to some of the methodologic issues present in studies, such as underpowered trials) as to the overall benefits of perioperative neuraxial anesthesia and analgesia on mortality, some credible data suggest that the use of both intraoperative neuraxial anesthesia and postoperative epidural analgesia may significantly lower mortality. A systematic review of randomized controlled trials (RCTs) comparing patients who received intraoperative neuraxial blockade or not included 141 trials (prior to January 1997) with 9,559 patients (19). The risk of mortality within 30 days of the surgical procedure was reduced by about a third in patients who received neuraxial anesthesia (103 deaths per 4,871 patients versus 144 deaths per 4,688 patients, odds ratio (OR) = 0.70, 95% confidence interval (CI): 0.54–0.90, p = 0.006) (Fig. 7-1) (19). A subsequent meta-analysis (20) indicated that the 30-day mortality after mixed orthopedic procedures was significantly lower with use of neuraxial anesthesia (versus general anesthesia) although many methodologic issues are present when interpreting the evidence. Despite the findings of some larger recent RCTs (21,22) that found no differences in mortality between neuraxial and general anesthesia, a meta-analysis suggested that high-risk patients may benefit from regional anesthesia (22).
Large-scale observational data also suggest that postoperative epidural analgesia may be associated with a significantly lower odds of death (23). A 5% random sample of the Medicare claims database from 1997 through 2001 was analyzed. Patients undergoing a variety of surgical procedures (colectomy, esophagectomy, gastrectomy, hysterectomy, liver resection, nephrectomy, pulmonary resection, radical retropubic prostatectomy, and total knee replacement) were stratified according to the presence (n = 12,780 subjects) or absence (n = 55,943) of a bill for postoperative epidural analgesia. After adjusting for comorbidities, age, gender, and hospital size, regression analysis revealed that the presence of postoperative epidural analgesia was associated with a significantly lower odds ratio for both 7-day (OR = 0.52; 95% CI: 0.38–0.73, p = 0.0001) and 30-day (OR = 0.74; 95% CI: 0.63–0.89, p = 0.0005) mortality (23). Not unexpectedly, there was a significantly lower mortality in patients who received postoperative epidural analgesia for higher-risk procedures (e.g., lung resection, colectomy) but no difference in mortality between patients who did or did not receive postoperative epidural analgesia in lower-risk procedures (e.g., total knee replacement, hysterectomy) undergone by patients with lower comorbidity indices.
Morbidity
Cardiovascular
Given that 8 million of the 27 million patients in the United States who have surgery every year have coronary artery disease or risk factors for cardiovascular disease, cardiac-related events are one of the most common causes of perioperative mortality (24). Within the United States alone, 1 million patients annually have perioperative cardiac complications (at an estimated cost of $20 billion) (25) and worldwide, approximately 5% of the surgical population will develop some type of perioperative cardiac complication (26).
Experimental data from animal studies suggest that thoracic epidural analgesia with local anesthetics (thoracic epidural analgesia; TEA) will provide a favorable balance of myocardial oxygen supply and demand particularly in patients with decreased cardiac reserve. Thoracic epidural analgesia may increase the diameter of stenotic epicardial coronary arteries in patients with coronary artery disease without changing the diameter of nonstenotic segments, and it does not induce any changes in coronary perfusion pressure, total myocardial blood flow, coronary venous oxygen content, or regional myocardial oxygen consumption (27). During myocardial ischemia, TEA may also attenuate an ischemia-induced decrease in myocardial pH (28), and it lessens the degree of regional ischemia induced by intracoronary injection of endothelin, a potent vasoconstrictor (29). Other animal studies indicate that TEA improves functional recovery from myocardial stunning and reduces infarct size after myocardial ischemia and reperfusion injury (30,31). In part through its favorable physiologic effects (blockade of sympathetic outflow, attenuation of stress response, increased coronary flow in stenotic segments) on the myocardium, TEA has been shown in animal studies to decrease the incidence of malignant ventricular arrhythmias and may actually protect against the generation of ventricular arrhythmias (32,33). It is important to remember that these physiologic benefits are provided only by TEA (a “catheter-incision congruent” technique) and not lumbar epidural analgesia (LEA; a “catheter-incision incongruent” technique) as there may be a compensatory increase in sympathetic activity above the level of blockade during lumbar epidural analgesia, which may not be particularly physiologically beneficial to the heart (34). Finally, in patients with multivessel ischemic heart disease, TEA increased myocardial blood flow in all vascular territories (35).
Several systematic reviews and meta-analyses examine the effects of neuraxial anesthesia and analgesia on cardiovascular events. Although the results of these meta-analyses might seem equivocal, they in fact are consistent when one asks whether a catheter-incision congruent technique was employed and whether the neuraxial technique persisted into the postoperative period, thus maximizing the physiologic benefits of neuraxial analgesia. One of the largest meta-analyses examined 141 RCTs describing 9,559 patients who were randomized to receive intraoperative neuraxial or general anesthesia (19). Although the patients who were randomized to receive neuraxial anesthesia had a significantly lower risk of 30-day mortality, there was no significant decrease in the risk of myocardial infarction (Fig. 7-2). It should be noted that the vast majority of patients received LEA or spinal anesthesia, which, as previously mentioned, may not provide the physiologic benefit of TEA.
Two meta-analyses examining the efficacy of postoperative epidural analgesia and cardiovascular events suggest a benefit for epidural analgesia. The first meta-analysis examined 11 RCTs comprising 1,173 patients undergoing a variety of surgical procedures in whom epidural analgesia was extended at least 24 hours into the postoperative period (36). There was no difference in the in-hospital death rate; however, the rate of myocardial infarction was significantly lower in those who received epidural analgesia (rate difference = –3.8%; 95% CI: –7.4%, –0.2%; p = 0.049). Subgroup analysis revealed that TEA but not LEA provided a significant reduction in the rate of myocardial infarction (rate difference = –5.3%; 95% CI: –9.9%, –0.7%; p = 0.04) (36), again supporting experimental data demonstrating physiologic cardiac benefits of TEA but not necessarily of LEA. The second meta-analysis examined 15 RCTs with 1,178 patients undergoing CAB surgery (37). There were no differences in the incidences of mortality or myocardial infarction; however, the use of TEA (versus systemic opioids) was associated with a significant reduction in the risk of dysrhythmias (24.8% versus 29.1%) (Table 7-1).
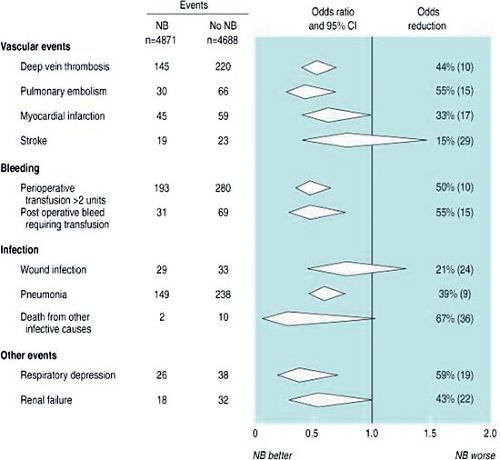 Figure 7-2. Subgroup analysis of Figure 7-1. Pooled estimates suggest that use of perioperative neuraxial anesthesia (compared to general anesthesia) is associated with a significantly lower odds of deep venous thrombosis, pulmonary embolism, bleeding complications, pneumonia, and respiratory depression. From Rogers et al. BMJ 2000;321:1493–1497, with permission. |
Pulmonary
Postoperative pulmonary complications (PPC) are a significant problem affecting approximately 10% of patients undergoing elective abdominal surgery (9) and are as common as cardiac complications in those undergoing noncardiac procedures (38,39). Compared to postoperative cardiac complications, PPC may be a better predictor of long-term postoperative mortality (39). Many factors, including uncontrolled postoperative pain, may result in decreased lung volumes, which in turn may contribute to the development of atelectasis and PPC.
Table 7-1 Effects of single-shot peripheral nerve blocks versus general anesthesia for ambulatory surgery | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
Epidural analgesia, particularly that using a local anesthetic–based solution, will confer superior analgesia compared to systemic opioids, including IV PCA (40,41). Segmental block from TEA may result in increased tidal volume and vital capacity related in part to improved pain control and interruption of the reflex inhibition of phrenic nerve activity, thus improving diaphragmatic activity (9). Thoracic epidural analgesia using bupivacaine 0.25% did not impair ventilatory mechanics and inspiratory respiratory muscle strength in patients with severe chronic obstructive pulmonary disease (42). Despite the presence of sympathetic blockade, TEA does not worsen airway obstruction in patients with severe chronic obstructive pulmonary disease (43). Although there are apparent benefits, the physiologic effects of TEA on respiratory muscle function are complex, with TEA possibly paralyzing other respiratory muscles such as the intercostals or abdominals (9).
Several meta-analyses indicate that the use of regional anesthesia and analgesia (versus general anesthesia and systemic opioids) may be associated with a significant decrease in the risk of PPC (19,37,44). An earlier meta-analysis of 48 RCTs demonstrated that the use of epidural analgesia using a local anesthetic-based regimen (versus systemic opioids) was associated with a significant decrease in PPC (44). These benefits were not seen with epidural opioids, intercostal blocks, or intrapleural analgesia (44). The subgroup analysis of a previously described meta-analysis (19) noted neuraxial anesthesia-analgesia reduce the odds of developing pneumonia by 39%,
compared to general anesthesia and systemic opioids. In addition, a more recent meta-analysis examining the perioperative use of TEA in patients undergoing CAB revealed that TEA was associated with a significant decrease in PPC (OR = 0.41; 95% CI: 0.28–0.61) (37). However, PPC might not be decreased with use of regional anesthesia-analgesia in all procedures: A meta-analysis of general versus regional anesthesia in patients undergoing hip fracture repair noted no difference in the risk of developing pneumonia (45). Finally, one systematic review suggested that IV PCA with opioids versus as-needed (PRN) systemic opioids was associated with a statistically significant decrease in PPC (OR = 0.93; 95% CI: 0.86–0.99) (46); however, two other meta-analyses examining the same topic (i.e., IV PCA versus PRN opioids) did not reveal any benefit of IV PCA in decreasing the incidence of PPC (47,48). Many methodologic issues contribute to the controversy of whether neuraxial anesthesia-analgesia decreases PPC, including the fact that there is no universally accepted definition of what constitutes a PPC.
compared to general anesthesia and systemic opioids. In addition, a more recent meta-analysis examining the perioperative use of TEA in patients undergoing CAB revealed that TEA was associated with a significant decrease in PPC (OR = 0.41; 95% CI: 0.28–0.61) (37). However, PPC might not be decreased with use of regional anesthesia-analgesia in all procedures: A meta-analysis of general versus regional anesthesia in patients undergoing hip fracture repair noted no difference in the risk of developing pneumonia (45). Finally, one systematic review suggested that IV PCA with opioids versus as-needed (PRN) systemic opioids was associated with a statistically significant decrease in PPC (OR = 0.93; 95% CI: 0.86–0.99) (46); however, two other meta-analyses examining the same topic (i.e., IV PCA versus PRN opioids) did not reveal any benefit of IV PCA in decreasing the incidence of PPC (47,48). Many methodologic issues contribute to the controversy of whether neuraxial anesthesia-analgesia decreases PPC, including the fact that there is no universally accepted definition of what constitutes a PPC.
Gastrointestinal
Postoperative ileus and decreased GI motility may cost the U.S. health care system several billions dollars annually (49). The pathophysiology of postoperative ileus and decreased GI motility is multifactorial and includes neurogenic (spinal, supraspinal adrenergic pathways), inflammatory (i.e., local inflammatory responses initiate neurogenic inhibitory pathways), and pharmacologic (e.g., opioids) mechanisms (10). Analgesic agents differ in their effects on GI motility and, as such, it is not unreasonable to expect a difference in rate of return of GI function with different analgesic regimens.
Experimental data consistently indicate that epidural analgesia shortens the duration of intestinal paralysis (50,51). Other experimental data indicate that epidural analgesia may actually increase the strength of colonic contractions without impairing anastomotic healing or increasing the risk of anastomotic leakage (52). Epidural analgesia has been shown to hasten recovery from postischemic paralytic ileus in a rat model (53) and to prevent endotoxin-induced gut mucosal injury in rabbits (54). In addition, there is also data suggesting that TEA may improve gastric microcirculation and minimize intestinal acidosis (55,56).
A systematic review of RCTs published in the Cochrane Library indicates consistently quicker return of GI function in subjects receiving epidural local anesthetic compared with those receiving systemic or epidural opioid (37 hours and 24 hours, respectively) (57). Other reviews also confirm that epidural analgesia enhances recovery after GI surgery (58,59). This benefit, however, was seen only in RCTs in which there was proper placement of the epidural catheter (i.e., “catheter-incision congruent analgesia,” in which the catheter tip placement corresponds with the incisional dermatome). In such studies, there was consistent benefit from (thoracic) epidural analgesia with regard to return of GI function (59). Use of a catheter-incision incongruent analgesia technique (e.g., LEA for upper abdominal surgery), even with local anesthetic, will obscure any possible benefit of epidural analgesia in providing an earlier return of GI function (59). It should be noted that the advantages of epidural analgesia with respect to return of GI function are greatest when used as part of a multimodal, accelerated rehabilitation care pathway (60) and that other factors, such as fluid therapy, may also influence postoperative GI motility (61).
Coagulation
In general, approximately 600,000 patients annually develop pulmonary embolism (PE), with 60,000 deaths resulting from this complication (11). During the perioperative period, coagulation-related complications may be an important cause of morbidity. It is widely recognized that DVT is a major complication following orthopedic procedures. In addition, PE is the most frequent cause of death associated with childbirth (11). In the absence of thromboembolic prophylaxis, the incidence of fatal PE ranges from 0.1% to 0.8% after general surgery, 0.3% to 1.7% after elective hip surgery, and 4% to 7% after emergency hip surgery (11).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree