Objectives/indications
Both premature infants and full-term infants may present with pathology distinct to the neonatal period. The majority of these entities occur in the critically ill neonate, in the neonatal intensive care unit (NICU).
This chapter is a brief overview of the application of point-of-care ultrasonography in this unique population. The majority of research originates from the radiology and surgical literature. However, worldwide, neonatologists are increasingly incorporating this technology into their practice at the bedside, in order to rapidly diagnose life-threatening conditions in the smallest and most fragile of patients.
Abdominal emergencies: Necrotizing enterocolitis
Background
Necrotizing enterocolitis (NEC) is an acquired condition of diffuse necrotic injury to the mucosal and submucosal layers of the bowel. It is the most common gastrointestinal emergency of all infants admitted to a NICU, and is the most serious gastrointestinal disorder that occurs during the neonatal period. Necrotizing enterocolitis affects mostly the extremely low-birth-weight (ELBW) infants, but can occur in full-term infants as well. The mortality rate for NEC is estimated to be 10–30%, or 11.5 to 12.3 per 100,000 infant deaths, greatly exceeding that of other gastrointestinal surgical disorders. Approximately 20% of babies with NEC will require surgical intervention, either for advanced disease or due to delay in diagnosis. Their mortality rate increases to 50%. Those that survive often have long-term problems including short-gut syndrome, growth impairment, and adverse neurodevelopmental outcomes.
The challenge with NEC is that most infants present with nonspecific symptoms, such as feeding intolerance and abdominal distension. For infants presenting with these complaints, feeds are often held and radiographs are obtained. Radiographic findings are important in the staging of NEC and determine the need for surgical intervention. The pathognomonic finding on plain radiographs is pneumatosis intestinalis. However, this finding is often absent or difficult to identify. Bohnhorst et al. showed that the sensitivity and specificity of pneumatosis intestinalis on abdominal radiographs was 75% and 91% respectively. When ultrasound and radiographs are combined, the sensitivity increases to 89%. Ultrasound imaging can identify portal venous gas, which is pathognomic for NEC. With new grading criteria for NEC, ultrasonography has a high specificity for the suspicion of NEC: approaching 100%. In addition, with the application of specific radiologic criteria, the diagnostic sensitivity reaches 90%.
Technique
Point-of-care questions
Transducer selection and orientation
Ultrasound imaging should be performed with the linear transducer (5–10 MHz, most commonly 7.5 MHz), with a small footprint. The higher frequency provides better resolution, while sacrificing penetration.
A suggested protocol is to scan the liver and portal vein in both the longitudinal and transverse views. Dördelmann et al. suggested a scan time of approximately 3 minutes, with a mean of 4.5 minutes (Figure 23.1a).
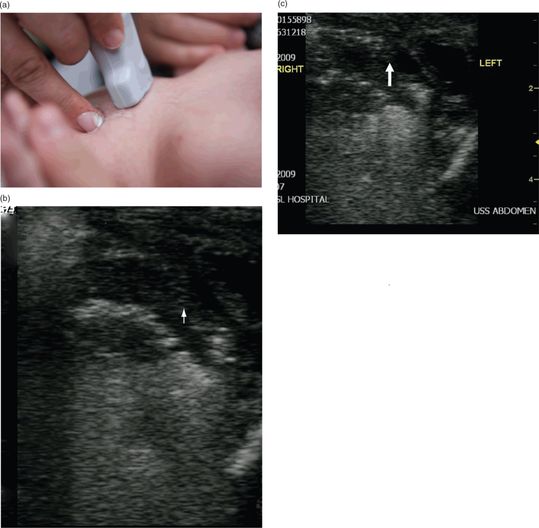
Figure 23.1 The evaluation for NEC. (a) Transducer placement. (b) Ultrasound image shows white dots with shadows (white arrow), indicative of air bubbles in the wall of the intestine. (c) Portal venous gas can also be visualized by ultrasound. Air bubbles appear hyperechoic (arrows). Ultrasound image courtesy of Michael Jones, MD RDMS FACEP.
Ultrasound imaging
Those babies suspected of having NEC may have an ultrasound evaluation, which can detect air bubbles moving through the wall of the intestine, otherwise known as pneumatosis intestinalis (Figure 23.1b). The ultrasound finding of air in the portal system can also be indicative of NEC. Sonographic findings of portal venous gas involve the trapping of air in the small branches of the portal vein inside the liver. This appears as dense granular echogenicities within the liver parenchyma. Portal venous gas may also be visualized as continuously streaming echogenic dots in the bloodstream. Doppler may also be helpful in detecting hyperemia or decreased blood flow in the wall of the intestine (Figure 23.2). These ultrasound findings, along with other clinical signs, can assist in making the diagnosis of NEC prior to bowel perforation and development of peritonitis.
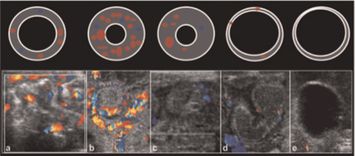
Figure 23.2 Doppler for necrotizing enterocolitis (NEC). The sequential changes in bowel wall thickness and perfusion in NEC, seen with transverse bowel sections (top) and color Doppler sonograms (bottom). (a) There is normal flow to normal bowel. The diagram shows normal bowel wall thickness and perfusion. (b) The changes of NEC are shown with bowel wall thickening and hyperemia. (c) The bowel wall thickening persists, but the perfusion has diminished. (d) As the process progresses, the mucosa starts to slough, and the bowel wall becomes much thinner; some perfusion persists. (e) Sloughing continues, the bowel wall becomes asymmetrically thinned, and blood flow ceases. Source (copyright): Epelman, M., Daneman, A., Navarro, O. M., et al. (2007) Necrotizing enterocolitis: review of state-of- the-art imaging findings with pathologic correlation. RadioGraphics 27: 285–305.
Abdominal emergencies: Bowel obstruction, malrotation with volvulus
Background
Neonatal bowel obstruction is a serious condition that can be complicated by intestinal perforation. Early radiologic studies in cases of neonatal bowel obstruction are needed to rule out intestinal malrotation with midgut volvulus. Intestinal malrotation is a congenital anomaly that involves an abnormal position of the small bowel: the small bowel is predominantly on the right side of the abdomen, the cecum is displaced, and the ligament of Treitz is displaced inferiorly and to the right. With malrotation, volvulus may occur, when there is a twisting of the bowel, that obstructs the mesenteric blood vessels, thereby causing intestinal ischemia. As many as 40% of infants with malrotation with volvulus present within the first week of life. It is often difficult to differentiate malrotation from other causes of intestinal obstruction such as intestinal atresia. Volvulus is fatal, if not surgically corrected within hours.
An Upper GI Series is the gold standard for evaluating for malrotation, and can determine the length of the mesenteric base. Ultrasonography can be helpful in diagnosing malrotation and volvulus. With malrotation, there is an abnormal position of the duodenojejunal junction, to the right of midline. Studies have suggested that an inversion of the superior mesenteric vein (SMV) and superior mesenteric artery (SMA) on ultrasound is diagnostic in nearly 100% of patients. In situations where the SMV lies anterior to the SMA, the incidence of malrotation decreases to 25–28%. Orzech et al. showed that, when compared with the gold standard of an Upper GI Series, ultrasonography had a false-positive rate of 15% and a false-negative rate of 2%. In those patients who had a negative ultrasound but positive Upper GI Series, there were no patients who had malrotation with midgut volvulus. Therefore, ultrasonography can serve as a good screening tool. Those patients with an abnormal ultrasound should have an upper GI study or go to the operating room, depending on their clinical presentation and consultation by a pediatric surgeon.
Technique
Point-of-care questions
Transducer selection and orientation
The evaluation for bowel obstruction, and malrotation with volvulus, differs a bit from other evaluations of the bowel. For the most part, ultrasound of the bowel is performed with a linear, high-frequency transducer (see Chapter 10). In contrast, the evaluation for bowel obstruction and for malrotation involves the lower-frequency transducers. For children, a low-frequency (2–5 MHz) curved-array transducer should be utilized; while a higher-frequency (4–8 MHz) micro-convex transducer should be utilized for newborns (Figure 23.3a). A methodic evaluation of the bowel should be utilized. The “mowing the lawn” approach has been suggested (Figure 23.3b).
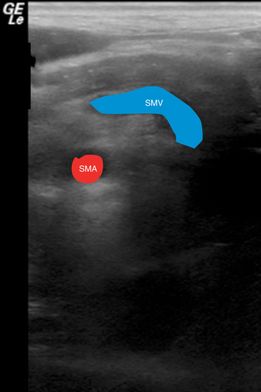
Figure 23.3 Malrotation with volvulus. Ultrasound image showing the “whirlpool” sign which involves the bowel and mesentery wrapping in a clockwise fashion around the superior mesenteric artery (SMA). Malrotation can also include the inversion of the SMA and SMV.
Ultrasound imaging
A distal small-bowel or large-bowel obstruction will show multiple, dilated loops of fluid-filled bowel throughout the abdomen (Figure 23.3c). Ultrasound can further distinguish between causes of bowel obstruction, including meconium ileus and ileal atresia. In meconium ileus, the bowel loops are filled with echogenic inspissated material. In contrast, in ileal atresia, bowel loops are filled with hypoechoeic or anechoic fluid. With intestinal atresia ultrasonography will demonstrate a dilated, fluid-filled intestinal loop adjacent to a very narrow loop of unused intestine.
Sonographic features of malrotation include the superior mesenteric vein positioned to the left of the superior mesenteric artery. The “whirlpool” sign can be visualized on ultrasound (Figure 23.4), which is pathognomonic for malrotation. It involves the bowel and mesentery wrapping in a clockwise fashion around the SMA. The “whirlpool” sign has a sensitivity of only 45.5% and negative predictive power of 71.4%, but specificity of 99% and PPV of 97.1%. Other sonographic features of malrotation with volvulus include duodenal dilatation with a tapering configuration, fixed midline bowel, and dilatation of the distal SMV.
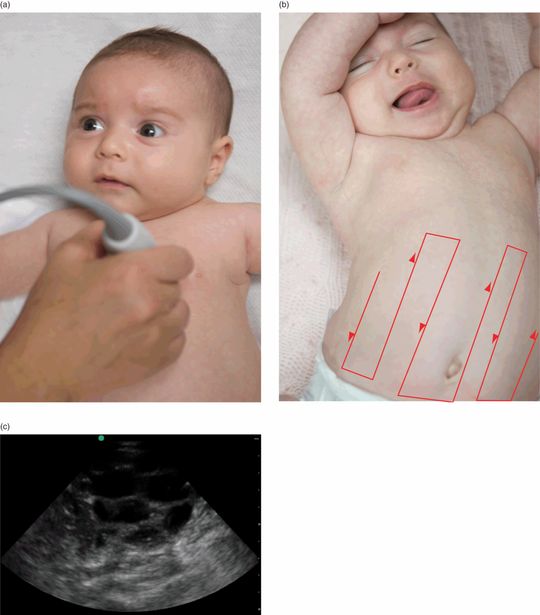
Figure 23.4 The evaluation for bowel obstruction. (a) Transducer placement. (b) A suggested approach for evaluating the bowel, referred to as “mowing the lawn.” (c) Ultrasound image showing dilated, fluid-filled loops of bowel consistent with a bowel obstruction.
Scanning tips
Pitfalls
- Bowel gas can obstruct the view of the SMA and SMV, and decrease the sensitivity of the sonographic demonstration of bowel.
- If the transducer is not maintained perfectly midline, there may be an oblique, inaccurate evaluation of the vessels.
Tips to improve scanning
Cranial ultrasound: IVH, hydrocephalus
Background
Preterm infants, particularly ELBW, are at high risk for many complications of prematurity. A sudden clinical deterioration can happen in the first few days of life, due to various causes. Severe intraventricular hemorrhage (IVH) is an important complication that can occur in ELBW infants. Its incidence is inversely related to gestational age, and occurs in 20–25% of low-birth-weight infants, and 1% of term infants. Intraventricular hemorrhage is due to impaired autoregulatory ability, which can result in rapid alterations in cerebral blood flow and large changes in cerebral blood pressure. These sudden changes can result in hemorrhage inside the lateral ventricles and hemorrhagic infarctions in the brain parenchyma adjacent to the lateral ventricles. The most severe form of IVH is a Grade IV intraventricular hemorrhage, which can manifest as a sudden clinical deterioration with metabolic acidosis, hypotension, apnea, and bradycardia. Other problems of prematurity include sepsis, pneumothorax, and patent ductus arteriosus. While these may all present with a similar clinical picture, it is important to differentiate between them, in order to best guide definitive treatment in an expeditious manner.
Following an intraventricular hemorrhage, one-third of neonates may develop post-hemorrhagic hydrocephalus. Hydrocephalus involves the dilatation of ventricles and eventually progresses to increased intracranial pressure, thereby causing damage to brain tissue and delayed neurodevelopment. Approximately 35% of neonates with post-hemorrhagic ventricular dilatation will need some sort of intervention, such as serial lumbar punctures or the placement of a ventriculoperitoneal shunt. Hydrocephalus may also be present in conditions such as congenital hydrocephalus and infections such as toxoplasmosis.
Ultrasound screenings are the benchmark for the detection of intraventricular hemorrhage and hydrocephalus in neonates. Ultrasonography can also identify pre-existing structural abnormalities or evidence of longstanding damage. It can further identify focal regions of hemorrhage and infarction. Ultrasound imaging results may assist in targeting other examinations such as MRI. Even once treatment has been initiated for hydrocephalus, serial cranial ultrasound measurements may be performed. Ultrasound has the potential to have a prognostic value with regards to neurodevelopmental outcomes in these high-risk neonates.
Anatomy
When performing ultrasonography of the neonatal brain, it is important to be familiar with the basic structures and ventricles in the coronal (Figure 23.6d) and sagittal orientations (Figure 23.7d).
Technique
Point-of-care questions
Transducer selection and orientation
Using a transducer that fits at the infant’s open anterior fontanel can easily yield the diagnosis. A phased-array transducer is often ideal for this type of imaging. In order to assess for the presence of a scalp hematoma, a linear, high-frequency transducer is utilized.
For a comprehensive cranial ultrasound examination, the brain should be evaluated at six coronal levels, in addition to the sagittal and parasagittal planes (Figure 23.5). Start by placing the transducer on the anterior fontanelle, with the indicator oriented towards the patient’s right side (Figure 23.6a). The resulting coronal ultrasound image will mimic the orientation as seen on tomography. Then, in order to obtain the sagittal view, rotate the indicator towards the patient’s anterior, front side (Figure 23.7a). Set the depth so that the base of the skull is visualized on the bottom of the ultrasound image. The depth is usually approximately 7 cm. Different levels can be obtained by angling the transducer. Then, rotate the transducer 90° in order to obtain the sagittal and parasagittal views.
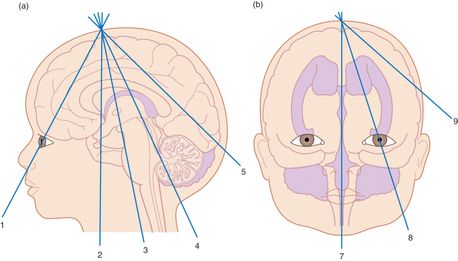
Figure 23.5 Scanning planes for cranial ultrasound examination. (a) The six coronal planes of scanning. (b) The sagittal and parasagittal planes of scanning. Artwork created by Emily Evans © Cambridge University Press.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








