Neonatal Sepsis and Congenital Infections
Camille Sabella
Approximately 10% of liveborn infants contract an infection in the neonatal period.
In comparison with older children and adults, neonates have less effective neutrophil function and natural killercell activity, lower antibody levels, and abnormal T-cell function and cytokine regulation. The relatively immunodeficient state of neonates, together with the increased survival of infants born ever more prematurely, contributes to a high risk for infection.
This chapter discusses three broad categories of neonatal infection:
Bacterial sepsis
Viral infections that mimic bacterial sepsis
Congenital (acquired in utero) infection
NEONATAL BACTERIAL SEPSIS
The overall incidence of neonatal bacterial sepsis is between 1 and 5/1000 live births. Classically, neonatal sepsis has been divided into early-onset and late-onset forms. Infants with early-onset sepsis manifest the symptoms within the first 5 days of life, usually have a mother with a history of obstetric complications, and invariably acquire the infection by the vertical transmission of organisms that normally colonize the maternal genital or gastrointestinal tract.
Infants with late-onset sepsis manifest the symptoms after day 5 of life. These neonates can be further divided into two distinct groups:
Healthy term neonates without risk factors for sepsis who present between day 5 of life and 3 months of age, usually with bacteremia and meningitis
High-risk hospitalized neonates in whom healthcare associated infections develop
The risk factors for bacterial sepsis associated with pregnancy and delivery include:
Prematurity and a low birth weight
Prolonged interval after rupture of the membranes (<18 hours)
Maternal peripartum fever
Depressed respiratory function at birth
Certain risk factors are associated with an intensive care environment, including the following:
Mechanical ventilation
Surgical/invasive procedures
Indwelling catheters
Total parenteral nutrition
Widespread use of broad-spectrum antimicrobial agents
H2 blockers
Premature, low-birth-weight infants are at the highest risk for infection; the incidence of early-onset sepsis is 4- to 25-fold higher in these infants than in full-term infants with a normal birth weight. The incidence of late-onset sepsis is also higher in premature infants because they are at risk for hospital-acquired infection.
Bacteriology of Neonatal Sepsis
The most common causes of early-onset neonatal sepsis in the United States are as follows:
Group B streptococci
Escherichia coli
Listeria monocytogenes
Although traditionally group B streptococci accounted for most cases of early-onset sepsis, there has been a recent decline in the incidence of early-onset neonatal group B streptococcal infection. This decline is a result of the successful implementation of routine maternal screening for, and maternal intrapartum antimicrobial prophylaxis against, this organism. Therefore, E. coli is now likely the
most common cause of early-onset sepsis in this country. Other, less common pathogens include viridans streptococci and nontypeable Haemophilus influenzae.
most common cause of early-onset sepsis in this country. Other, less common pathogens include viridans streptococci and nontypeable Haemophilus influenzae.
Group B streptococci and L. monocytogenes remain important causes of late-onset neonatal sepsis in previously healthy infants. In high-risk neonates who are hospitalized, the most important causes of late-onset sepsis, in decreasing order of relative frequency, are as follows:
Coagulase-negative staphylococci
Gram-negative bacilli (E. coli, Klebsiella spp., Enterobacter spp.)
Candida species
Staphylococcus aureus
Enterococci
Coagulase-negative staphylococci are the most common cause of late-onset neonatal sepsis in hospitalized high-risk neonates, accounting for almost 50% of hospital-acquired infections in neonatal intensive care settings.
Clinical Manifestations
The clinical features of neonatal sepsis are subtle and nonspecific. Respiratory distress, lethargy, an unstable temperature, apnea, jaundice, feeding intolerance, and tachycardia are common. Regardless of the specific pathogen causing the sepsis, the clinical manifestations are similar and also do not differ from those of noninfectious illnesses in neonates. Finally, it is important to remember that the characteristics of neonatal meningitis are indistinguishable from those of sepsis. The specific epidemiologic and clinical features of disease caused by the most common pathogens are discussed in the subsequent text.
Group B Streptococci
Group B streptococci are important causes of both earlyand late-onset sepsis, with an incidence of 0.3/1000 live births for each. These organisms are frequent inhabitants of the maternal genital and gastrointestinal tracts; 15% to 40% of all pregnant women are colonized with group B streptococci. This colonization may be constant or intermittent, and the presence of colonization in a pregnant woman in one pregnancy does not predict colonization in another pregnancy. Although 50% to 70% of mothers in whom organisms are colonized transmit the organisms to their infants if intrapartum antimicrobial prophylaxis is not given, early-onset group B streptococcal sepsis develops in only 1% to 2% of the colonized infants. The risk increases dramatically in infants with any high-risk gestational factors, which include:
Prematurity (Increased sevenfold for babies with birth weight <2500 g and gestation <37 weeks)
Prolonged rupture of membranes (>18 h)
Chorioamnionitis/maternal fever
Group B streptococcal bacteriuria
Previous Group B Streptococcal-infected infant
Deficiency of transplacentally acquired serotype specific Group B Streptococcal capsular polysaccharide serum antibody
The most common manifestations of early-onset group B streptococcal sepsis are pneumonia and apnea, and they usually occur within the first 24 hours of life. Septic shock occurs in 25% of affected infants, and meningitis in 5% to 10%. The radiographic appearance of group B streptococcal pneumonia is indistinguishable from that of respiratory distress syndrome or other infections. The case fatality rate for early-onset group B streptococcal sepsis is 10% to 15%.
Late-onset group B streptococcal sepsis usually affects term infants between 1 and 12 weeks of age who have had an unremarkable early neonatal history. Bacteremia and purulent meningitis are the most common features, although focal infections such as osteomyelitis and adenitis can also occur. The case fatality rate is 2% to 6%; however, long-term neurologic sequelae develop in 25% to 50% of the survivors of group B streptococcal meningitis.
Escherichia coli
The incidence of neonatal sepsis caused by E. coli is approximately 1/1000 live births. Most of these infants present within the first few days of life. The K1 capsular antigen of the organism is associated with neonatal infection, and the antigen is detected in 80% of the cases of meningitis.
Vertical transmission appears to be the major route by which infants acquire the organism. Infants with galactosemia are particularly susceptible to E. coli infection.
The clinical features of sepsis and meningitis caused by E. coli are similar to those of infections caused by group B streptococci and other pathogens. It should be pointed out that gram-negative neonatal meningitis is associated with a higher incidence of brain abscess. Therefore, the cerebrospinal fluid (CSF) of an infant (that has not been sterilized with appropriate antibiotics) with gram-negative meningitis should prompt a search for a brain abscess. A gram-negative organism that causes neonatal meningitis, Citrobacter koseri (formerly diversus), is strongly associated with the development of brain abscess. The case fatality rate for sepsis and meningitis caused by E. coli is 15% to 25%, but significant neurologic sequelae develop in 30% to 50% of survivors.
Listeria monocytogenes
L. monocytogenes is a gram-positive bacillus that is rarely associated with sporadic neonatal sepsis, but it has been associated with several food-borne outbreaks of perinatal disease that have resulted in substantial morbidity and mortality.
As in group B streptococcal infection, early- and late-onset neonatal diseases have been described. The incidence
of prematurity and gestational complications is relatively high in infants with early-onset disease. Most, but not all, mothers of these infants have experienced an influenza-like illness, representing maternal listeriosis, during the third trimester of pregnancy. Late-onset infection affects full-term neonates of uncomplicated pregnancies.
of prematurity and gestational complications is relatively high in infants with early-onset disease. Most, but not all, mothers of these infants have experienced an influenza-like illness, representing maternal listeriosis, during the third trimester of pregnancy. Late-onset infection affects full-term neonates of uncomplicated pregnancies.
Pneumonia, septicemia, and meningitis are the most common features of early-onset disease. An erythematous rash, characterized by widespread granulomatous lesions of the skin and other organs, may accompany severe disease, and is termed granulomatosis infantisepticum. The blood monocyte count is elevated in approximately 50% of infected infants.
Infants with late-onset infection typically present with bacteremia and meningitis. They may present anytime between the first and eighth weeks of life. CSF characteristics of infants with meningitis include a polymorphonuclear (not mononuclear) leukocytosis with an elevated protein level; 60% of infants have a normal CSF glucose level.
The mortality rate for early-onset listeriosis is approximately 25%, whereas that for late-onset disease is approximately 5%.
Diagnosis
The isolation of an organism from the blood or CSF provides definitive evidence of bacterial sepsis. The utility of rapid diagnostic tests such as latex particle agglutination to detect group B streptococcal and E. coli infections is limited by poor sensitivity and specificity. Because 25% to 30% of neonates with bacterial sepsis also have meningitis, a lumbar puncture should be performed whenever sepsis is documented. Even in the absence of a blood culture that is positive for bacteria, examination of the CSF should be strongly considered whenever sepsis is suspected because the blood cultures of 10% to 15% of neonates with meningitis are negative for the bacteria. The hematologic findings associated with neonatal sepsis include an elevated ratio of immature neutrophils to total neutrophils (>0.2), neutropenia, an elevated total neutrophil count, and thrombocytopenia.
Therapy
Antimicrobial therapy for early-onset neonatal sepsis must include coverage for group B streptococci, E. coli, and L. monocytogenes. Administration of ampicillin/gentamicin provides such coverage. Although gentamicin has no activity against group B streptococci and L. monocytogenes, when it is administered with penicillin, the combination is synergistic.
Antimicrobial coverage for late-onset infection acquired in the neonatal intensive care nursery setting usually includes a combination of oxacillin or vancomycin to provide coverage against gram-positive organisms, including coagulase-negative staphylococci, and an aminoglycoside or thirdgeneration cephalosporin to provide broad gram-negative coverage. Once the organism has been recovered and sensitivity results are available, the regimen can be re-evaluated.
Initial antimicrobial therapy for an infant with late-onset sepsis or meningitis that has not been acquired nosocomially usually includes a combination of ampicillin and an aminoglycoside or third-generation cephalosporin, such as cefotaxime. Ampicillin is required in this situation to cover the possibility of L. monocytogenes infection, against which cephalosporins have no activity.
Prevention
Most preventive efforts are focused on group B streptococci infection. Currently, group B streptococcal screening with vaginal and rectal cultures is recommended for all pregnant women at 35 to 37 weeks’ gestation, with the exception of mothers who have had group B streptococcal bacteriuria during the current pregnancy or those having had a previous infant with group B streptococcal disease. Mothers who are shown to be carriers of group B streptococci during the current pregnancy should receive intrapartum prophylaxis, unless a planned cesarean delivery is performed in the absence of labor or membrane rupture. Maternal intrapartum antimicrobial prophylaxis is also indicated for mothers with:
Group B streptococcal bacteriuria during current pregnancy
Previous infant with group B streptococcal disease
If the group B streptococcal status of the mother is unknown at the time of delivery, maternal intrapartum prophylaxis is indicated if any of the following are present:
Delivery at less than 37 weeks gestation
Membranes ruptured for 18 hours or longer
Intrapartum fever (temperature ≥38°C [100.4°F])
VIRAL SEPSIS
Important viral agents acquired at birth (natal infection) or after birth but during the neonatal period (postnatal infection) that may mimic bacterial neonatal sepsis include:
Herpes simplex virus (HSV)
Enteroviruses
Cytomegalovirus (CMV)
Herpes Simplex Virus
Neonatal HSV infection is the most feared consequence of gestational genital HSV infection. Because HSV infection closely mimics bacterial sepsis, a delayed diagnosis is common, and despite the availability of antiviral therapy, the associated mortality and morbidity remain high.
Most neonates acquire the virus from an infected maternal genital tract at the time of delivery. HSV 2 accounts for most cases of neonatal HSV infection; approximately 30% of the cases are caused by HSV 1. Viral transmission can follow either primary or recurrent maternal infection. The transmission rate during primary maternal infection is estimated to be 35% to 50%, but 3% to 5% during recurrent maternal infection. The higher rates are likely the consequence of a high viral titer, involvement of the cervix, and the lack of transplacental antibody in the primary maternal infection. Most neonates with HSV infection are born to asymptomatic mothers with no past history of genital HSV infection. This finding is explained by the high prevalence of “silent” maternal genital HSV infection.
There are three clinical forms of neonatal HSV infection (Table 22.1):
Disseminated disease
Central nervous system (CNS) disease
Localized disease involving the skin, eyes, and/or oral mucosa (SEM)
Infants with disseminated disease usually present during the first 2 weeks of life with nonspecific symptoms. Irritability, apnea, poor feeding, unstable temperature, jaundice, hepatosplenomegaly, disseminated intravascular coagulation, hepatic failure, seizures, and shock are common features. A vesicular rash occurs in about 60% of the cases but is often absent at the time of presentation. Seizures occur in 22% of patients with disseminated disease. Even with appropriate antiviral therapy, the mortality rate associated with this form of HSV infection is 30%.
Neonates with disease localized to the CNS most commonly present between the second and third weeks of life, usually with lethargy, fever, and seizures. CSF pleocytosis with an elevated CSF protein is the rule. Approximately 65% of these infants have skin vesicles at any time during the course of the disease. It is important to remember that neonatal HSV causes diffuse encephalitis, without localization to the temporal lobes, unlike HSV encephalitis beyond the neonatal period. The mortality rate for this form of disease is 5%, but at least two thirds of the survivors have long-term neurologic sequelae.
TABLE 22.1 CLINICAL FEATURES OF THE THREE MAJOR FORMS OF NEONATAL HERPES SIMPLEX VIRUS INFECTION | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
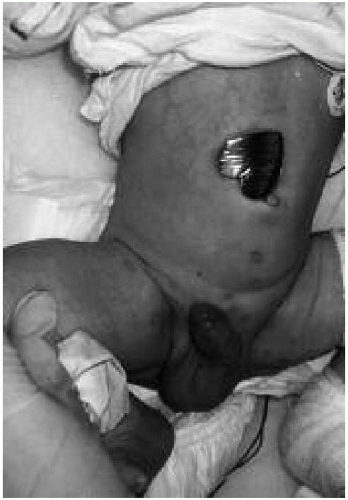 Figure 22.1 A neonate with multiple vesicular lesions on the abdomen and perineum. Results of direct fluorescent antibody testing and viral culture of the vesicular fluid were positive for herpes simplex virus type 1. (See color insert.) |
Neonates with HSV disease localized to the SEM usually present between 1 and 2 weeks after birth with vesicular lesions, forming singly or in clusters, on an erythematous base (Fig. 22.1). Ocular involvement, in the form of keratitis, conjunctivitis, or chorioretinitis, occurs in 10% to 15% of these infants. Patients with SEM disease have the best prognosis; infants treated early in the course with antiviral agents uniformly survive. However, cutaneous lesions may recur in 10% to 15% of treated infants during the first year of life, and they are at risk for long-term neurologic sequelae. If SEM disease is not treated, it progresses to the disseminated and CNS form.
The diagnosis of neonatal HSV infection is difficult, especially in the absence of vesicular lesions. Therefore, a high index of clinical suspicion is warranted in any neonate in whom sepsis is suspected, especially in the setting of negative bacterial cultures. Viral culture is the most reliable method of making the diagnosis. The virus can be isolated from cutaneous lesions or from the nasopharynx, CSF, conjunctivae, or maternal genital tract. When vesicular lesions are present, a direct fluorescent antibody test can provide a rapid diagnosis. Polymerase chain reaction testing for HSV DNA in the CSF is sensitive and is the diagnostic method of choice for establishing CNS disease.
Parenteral acyclovir is the antiviral agent of choice in the treatment of neonatal HSV infection. The currently recommended dosage of acyclovir is 60 mg/kg per day given in three divided doses. The duration of the therapy is 14 days for SEM disease and 21 days for disseminated or CNS disease. Neonates treated with this dosage of acyclovir should be monitored closely for the development of neutropenia. It must be stressed that despite appropriate and timely antiviral therapy, the mortality and morbidity rates associated with these infections remain high.
Enteroviruses
Nonpolio enteroviruses are a common cause of benign febrile infections in older infants and children but have the potential to cause more severe infections in neonates. These viruses are transmitted by the fecal-oral and oral-oral routes, and newborns most commonly acquire them during the birth process. In temperate climates, enteroviral diseases are most common in the summer and fall. Echoviruses 9, 11, 30, and Coxsackie virus B are the most common causes of enteroviral neonatal disease.
Most neonatal enteroviral infections are mild and cause nonspecific symptoms. However, 20% of infections are severe and life threatening, and these occur in neonates who acquire the infection without the maternal antibody. Fever, feeding intolerance, abdominal distension, and irritability are the common presenting features. Diarrhea, vomiting, shock, disseminated intravascular coagulation, hepatomegaly, jaundice, and apnea may follow. A rash is present in 40% of the infants; macular and maculopapular rashes are most common, but petechial and vesicular exanthems have also been described. Hepatitis, hepatic necrosis, myocarditis (especially with coxsackievirus B), and meningoencephalitis may be evident.
Distinguishing the enteroviral illness from HSV infections and bacterial sepsis is difficult. The following features are more suggestive of an enteroviral infection:
Recent maternal history of a febrile illness and abdominal pain
Lack of obstetric complications
Illness in the summer or fall
Presence of myocarditis or hepatitis
Isolation of the virus from the nasopharynx, throat, stool, rectal swab, blood, urine, or CSF confirms the diagnosis. Polymerase chain reaction assays for the presence of enterovirus RNA on CSF is more sensitive than the viral culture in infants with meningoencephalitis. The management of these infants relies on meticulous intensive care. A neonate with myocarditis, encephalitis, or hepatic involvement has a poor prognosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





