Neonatal Respiratory Failure
Narayan Prabhu Iyer
Philippe S. Friedlich
Elisabeth L. Raab
Rangasamy Ramanathan
Istvan Seri
KEY POINTS
 Respiratory disorders in newborn infants are caused by diseases unique to the neonatal population and that often result in long-term respiratory complications.
Respiratory disorders in newborn infants are caused by diseases unique to the neonatal population and that often result in long-term respiratory complications. Neonatal respiratory disorders could be developmental, congenital, or acquired; respiratory problems associated with prematurity are the most common cause for respiratory failure in newborn infants.
Neonatal respiratory disorders could be developmental, congenital, or acquired; respiratory problems associated with prematurity are the most common cause for respiratory failure in newborn infants. Management of respiratory distress syndrome (RDS) has changed significantly in the last two decades, with increasing emphasis on noninvasive respiratory support.
Management of respiratory distress syndrome (RDS) has changed significantly in the last two decades, with increasing emphasis on noninvasive respiratory support. Current research has been focused on refining the indication for surfactant and postnatal steroids use and to identify the optimal pulse oximetry range for preterm infants that minimizes the risk for retinopathy of prematurity (ROP) without increasing the mortality.
Current research has been focused on refining the indication for surfactant and postnatal steroids use and to identify the optimal pulse oximetry range for preterm infants that minimizes the risk for retinopathy of prematurity (ROP) without increasing the mortality. Management of persistent pulmonary hypertension of newborn (PPHN) has improved dramatically after the introduction of inhaled nitric oxide. However, PPHN unresponsive to nitric oxide remains a challenge, and newer treatments such as sildenafil and bosantan are increasingly being used to improve outcomes in such patients.
Management of persistent pulmonary hypertension of newborn (PPHN) has improved dramatically after the introduction of inhaled nitric oxide. However, PPHN unresponsive to nitric oxide remains a challenge, and newer treatments such as sildenafil and bosantan are increasingly being used to improve outcomes in such patients.Respiratory disease accounts for a significant proportion of admissions to the neonatal intensive care unit (NICU) and the pediatric intensive care unit (PICU). The unique aspects of neonatal respiratory physiology place the newborn at high risk for developing respiratory morbidity. Respiratory symptoms in the neonate, such as tachypnea, cyanosis, grunting, flaring, or retractions, may be due to a primary pulmonary process or  may be the initial clinical manifestation of a nonrespiratory process, such as inborn errors of metabolism, polycythemia,
may be the initial clinical manifestation of a nonrespiratory process, such as inborn errors of metabolism, polycythemia,  congenital heart disease, and sepsis. Respiratory failure (due to RDS in the preterm neonate or due to a congenital infection, meconium aspiration syndrome, or congenital anomalies in the term infant) is a major cause of morbidity and mortality in the neonatal period and often has long-term implications for the health of the child. Causes of respiratory failure in a neonate can be categorized as respiratory problems associated with transition around the time of birth, respiratory diseases related to prematurity, cardiac causes, congenital conditions, and, finally, acquired respiratory problems.
congenital heart disease, and sepsis. Respiratory failure (due to RDS in the preterm neonate or due to a congenital infection, meconium aspiration syndrome, or congenital anomalies in the term infant) is a major cause of morbidity and mortality in the neonatal period and often has long-term implications for the health of the child. Causes of respiratory failure in a neonate can be categorized as respiratory problems associated with transition around the time of birth, respiratory diseases related to prematurity, cardiac causes, congenital conditions, and, finally, acquired respiratory problems.
 may be the initial clinical manifestation of a nonrespiratory process, such as inborn errors of metabolism, polycythemia,
may be the initial clinical manifestation of a nonrespiratory process, such as inborn errors of metabolism, polycythemia,  congenital heart disease, and sepsis. Respiratory failure (due to RDS in the preterm neonate or due to a congenital infection, meconium aspiration syndrome, or congenital anomalies in the term infant) is a major cause of morbidity and mortality in the neonatal period and often has long-term implications for the health of the child. Causes of respiratory failure in a neonate can be categorized as respiratory problems associated with transition around the time of birth, respiratory diseases related to prematurity, cardiac causes, congenital conditions, and, finally, acquired respiratory problems.
congenital heart disease, and sepsis. Respiratory failure (due to RDS in the preterm neonate or due to a congenital infection, meconium aspiration syndrome, or congenital anomalies in the term infant) is a major cause of morbidity and mortality in the neonatal period and often has long-term implications for the health of the child. Causes of respiratory failure in a neonate can be categorized as respiratory problems associated with transition around the time of birth, respiratory diseases related to prematurity, cardiac causes, congenital conditions, and, finally, acquired respiratory problems.DEVELOPMENTALLY REGULATED DISORDERS: RESPIRATORY CONDITIONS ASSOCIATED WITH PREMATURE LUNG
Respiratory Distress Syndrome
Respiratory distress syndrome (RDS) due to surfactant deficiency is a significant life-threatening condition that is seen primarily in preterm infants. The incidence and severity of RDS is inversely proportional to gestational age (GA) (Fig. 47.1). RDS occurs in about 50% of infants born at less than 29 weeks and in 25% of infants born after 29 weeks of gestation. Despite significant improvements in perinatal care practices, the incidence of prematurity has not decreased significantly over recent years. One out of eight babies is born preterm, and this translates into ˜480,000 preterm births each year in the United States. Fortunately, with the maternal administration of prenatal steroids to accelerate fetal organ development in general and lung maturity and surfactant release in particular, the incidence and severity of RDS has decreased by nearly 50%. Neonatal outcomes in symptomatic infants with RDS have been significantly improved by the introduction of surfactant. The combination of prenatal steroid followed by postnatal surfactant therapy has resulted in a significant reduction in the mortality and morbidity in preterm infants with RDS (1).
Operational Definition of Respiratory Distress Syndrome
RDS is the collection of symptoms associated with deficiency of pulmonary surfactant. Surfactant deficiency leads to increased alveolar surface tension, subsequent alveolar collapse, decreased pulmonary compliance, and reduced functional residual capacity (FRC). The resultant symptoms include clinical findings of respiratory distress such as retractions, grunting, nasal flaring, and cyanosis. These clinical symptoms are nonspecific for surfactant deficiency. The need for supplemental oxygen, positive pressure ventilation (PPV), and use of radiographic criteria (diffuse haziness and air bronchograms) makes the definition more specific. Radiologic features may be altered by treatment of RDS such as provision of PPV and early surfactant administration. Recent trials have revealed a subgroup of premature infants, some very premature, who do not have “significant” surfactant deficiency (2,3,4). One of the arms of these randomized trials had infants, including
extremely premature infants (<28 weeks), being stabilized on CPAP after birth. In these trials, 33%-62% of the infants stabilized on CPAP completed the study without receiving surfactant. This finding suggests that these infants did not have “significant” surfactant deficiency but fulfill the clinical criteria for the diagnosis of RDS.
extremely premature infants (<28 weeks), being stabilized on CPAP after birth. In these trials, 33%-62% of the infants stabilized on CPAP completed the study without receiving surfactant. This finding suggests that these infants did not have “significant” surfactant deficiency but fulfill the clinical criteria for the diagnosis of RDS.
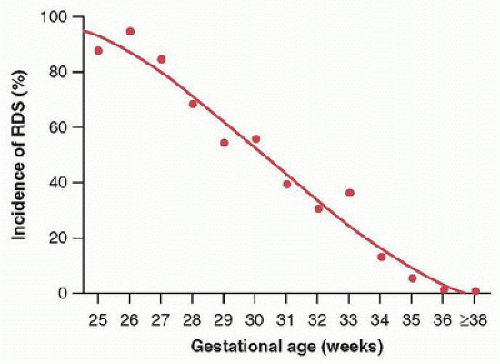 FIGURE 47.1. Incidence of RDS in relation to gestational age at birth (Reused from Robertson PA, Sniderman SH, Laros RK Jr, et al. Neonatal morbidity according to gestational age and birth weight from five tertiary care centers in the United States, 1983 through 1986. Am J Obstet Gynecol 1992;166(6, pt 1):1629-41; discussion 1641-5.) |
Pathophysiology of Respiratory Distress Syndrome
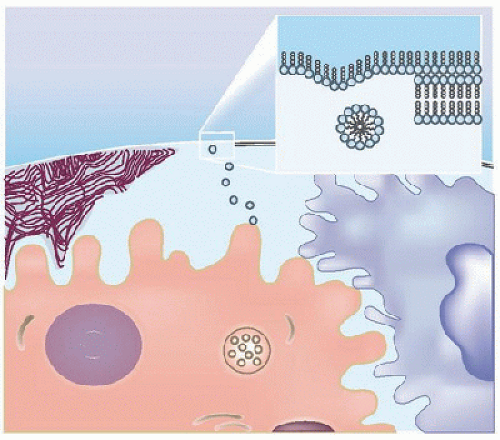
Surfactant Synthesis, Metabolism, and Function. The newborn lung provides an extensive surface area for gas exchange, ˜1 m2/kg body weight (70 m2 in an adult lung). Pulmonary surfactant coats the lung surface at the air-liquid interface in the alveoli and maintains a very low surface tension that prevents collapse of the lung, especially at the end of expiration when the alveolar radius is at its lowest. Surfactant is primarily composed of phospholipids (80%-90%) and surfactant proteins (SP) SP-A, SP-B, SP-C, and SP-D. Dipalmitoyl phosphatidylcholine (DPPC) constitutes the major phospholipid. Surfactant is synthesized by the type II pneumocytes lining the alveoli and is stored as lamellar bodies. Lamellar bodies enter the alveoli by exocytosis and transform into tubular myelin. Under the influence of SP-A, which is cosecreted along with the lamellar bodies from type II pneumocytes, tubular myelin unravels to form mono- and multilayers of phospholipids rich in SP-B and SP-C. The spread and adsorption of phospholipids at the air-liquid interphase is dependent on SP-B and to a lesser extent on SP-C (Fig. 47.2) (5). Surfactant reduces surface tension at the air-liquid interphase, thereby improving pulmonary compliance. Deficiency of surfactant is associated with heterogeneous expansion of alveoli. According to the law of Laplace, the retractive forces are higher in the smaller alveoli compared with the bigger alveoli, resulting in further collapse of the smaller alveoli. Surfactant reduces surface tension independent of alveolar size, thereby equalizing surface tension forces across the lung, resulting in more uniform alveolar expansion (6). Catabolism of secreted surfactant is primarily regulated by lung macrophages and SP-A. Surfactant metabolism is highly regulated; remnants of phospholipids, SP-A, SP-B, and SP-C are taken up almost entirely and recycled or degraded by type II pneumocytes (7,8,9). The composition of surfactant across different mammalian species is fairly constant, making it possible to modify surfactant from one species and use it in another species.
Consequences of Surfactant Deficiency. Infants with RDS usually have surfactant lipid pools <10 mg/kg as compared with normal term infants, who have surfactant lipid pools of 100 mg/kg (10). In a preterm infant, this results in very low pulmonary compliance after delivery and an inability to maintain FRC. Because surfactant is responsible for uniform alveolar expansion, surfactant deficiency results in a heterogeneous disease with underaerated alveolus emptying into adjacent overdistended alveolus. The neonate has respiratory distress, hypoxemia, and may develop air leak syndrome. Introduction of PPV using high pressures in this setting, while lifesaving, causes lung injury and inflammation. Pulmonary edema inactivates and dilutes the remaining surfactant, which promotes a vicious cycle.
Management of Respiratory Distress Syndrome
 Management of RDS has revolved around the use of lung distending pressure and replacement of pulmonary surfactant. Below is a brief description of our current state of knowledge and practice.
Management of RDS has revolved around the use of lung distending pressure and replacement of pulmonary surfactant. Below is a brief description of our current state of knowledge and practice.Surfactant Therapy for Respiratory Distress Syndrome. Surfactant therapy has been extensively evaluated in randomized, controlled trials in neonatal medicine and has become the standard of care for preterm infants with RDS. Surfactant therapy has decreased the mortality and incidence of pneumothorax in preterm infants with RDS (11).
Timing of Surfactant. Surfactant therapy has been used prophylactically in all preterm infants at risk for RDS, as well as more selectively in infants with established RDS. In the 1990s,
a time when antenatal steroids and stabilization of preterm infants on nasal continuous positive airway pressure (NCPAP) were not routine, many trials comparing prophylactic versus selective surfactant therapy were done. Meta-analysis of these trials supported the use of prophylactic surfactant (surfactant given within 15 minutes of birth), with 31% lower neonatal mortality in infants born before 32 weeks of gestation (12). With increasing evidence of lung injury associated with invasive mechanical ventilation, more recent trials have focused on the impact of avoiding routine intubation (and, therefore, avoiding routine surfactant) with initial stabilization of preterm infants on NCPAP at delivery in comparison with routine intubation and surfactant administration (2,3,4,13,14,15,16,17). A meta-analysis of two recent studies, where the use of antenatal steroids and postnatal CPAP was common, demonstrated a small but statistically significant increase in the risk of bronchopulmonary dysplasia (BPD) or death with the prophylactic administration of surfactant when compared with selective use of surfactant in infants stabilized on NCPAP after birth (relative risk 1.12; 95% CI



a time when antenatal steroids and stabilization of preterm infants on nasal continuous positive airway pressure (NCPAP) were not routine, many trials comparing prophylactic versus selective surfactant therapy were done. Meta-analysis of these trials supported the use of prophylactic surfactant (surfactant given within 15 minutes of birth), with 31% lower neonatal mortality in infants born before 32 weeks of gestation (12). With increasing evidence of lung injury associated with invasive mechanical ventilation, more recent trials have focused on the impact of avoiding routine intubation (and, therefore, avoiding routine surfactant) with initial stabilization of preterm infants on NCPAP at delivery in comparison with routine intubation and surfactant administration (2,3,4,13,14,15,16,17). A meta-analysis of two recent studies, where the use of antenatal steroids and postnatal CPAP was common, demonstrated a small but statistically significant increase in the risk of bronchopulmonary dysplasia (BPD) or death with the prophylactic administration of surfactant when compared with selective use of surfactant in infants stabilized on NCPAP after birth (relative risk 1.12; 95% CI
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree