Key Clinical Questions
What are the most common etiologies of pericarditis and myocarditis?
How are pericarditis and myocarditis diagnosed?
Which imaging modality is most accurate in diagnosing myocarditis?
What are the evidence-based physical examination, radiographic, electrocardiographic, and imaging findings used in the diagnosis of cardiac tamponade, and how is it managed?
What are the differences in physical examination, testing results, and treatment of constrictive pericarditis versus cardiac tamponade?
Myocarditis
Acute myocarditis is an inflammatory infiltration of the myocardium. The clinical sequelae of this disease include subclinical disease to fulminant cardiogenic shock.
Acute myocarditis can present a challenging diagnostic dilemma. Traditionally, the diagnosis of myocarditis is made by endomyocardial biopsy. In acute myocarditis, biopsy shows areas of necrosis with a lymphocytic infiltration. However, endomyocardial biopsy is subject to procedural risks, sampling bias, and poor sensitivity. Noninvasive cardiac magnetic resonance imaging (MRI) has become an alternative mechanism for diagnosing myocarditis.
The most common cause of myocarditis is a viral pathogen. However, other infectious, autoimmune, and toxic causes occur as well. Initial studies in the latter half of the 20th century indicated Coxsackie B virus as the frequent causative agent in endomyocardial biopsies. However, since the 1990s adenovirus has emerged as the more frequent viral agent. Since that time, parvovirus and other viruses have been seen in the biopsy results of patients with myocarditis. Other viral causes of acute myocarditis have been reported in patients with hepatitis C virus (HCV), Epstein-Barr virus, and cytomegalovirus (CMV).
Infectious etiologies other than viruses have been implicated as a cause of myocarditis, including mycoplasma and Borrelia burgdorferi (Lyme disease). In developing countries, Trypanosoma cruzi has been known to infect the myocardium causing Chagas disease.
The clinical presentation of patients with myocarditis can range from mild dyspnea and chest discomfort to fulminant cardiogenic shock. Patients may report a viral prodrome in the weeks preceding presentation, including fevers, respiratory, or GI symptoms. According to the European Study of Epidemiology and Treatment of Inflammatory Heart Disease, patients with myocarditis present with dyspnea (72%), chest pain (32%), and arrhythmias (18%). Patients may also present with symptoms related to congestive heart failure, including fatigue, orthopnea, paroxysmal nocturnal dyspnea, palpitations, and syncope.
As an infiltrative process, the amount of affected myocardium can be highly variable in myocarditis. Some patients may present with ventricular arrhythmias if the conducting system is involved while other patients may present with a dilated cardiomyopathy.
The physical examination of patients with acute myocarditis may correlate with the patients’ symptoms. In patients with mild symptoms, the physical examination may be relatively normal. However, patients with severe myocarditis may appear in cardiogenic shock. Diaphoresis, rales, jugular venous distention, hypotension, and peripheral edema can all be seen in patients with myocarditis.
The electrocardiogram (ECG) in myocarditis may show sinus tachycardia and nonspecific ST-segment changes and T-wave inversion. ST-segment elevation and Q-waves that suggest acute myocardial ischemia can occur in myocarditis. Patients may also present with bundle-branch blocks, atrioventricular block, or ventricular tachyarrhythmias. An associated pericarditis may be present in patients with myocarditis, so called myopericarditis, which can also cause diffuse ST-segment elevation in addition to PR-segment depression.
ECG findings in acute pericarditis include:
|
Patients with myocarditis can have elevated biomarkers, such as troponin-I, CK-MB, and CPK. Troponin-I levels are more commonly elevated than other cardiac biomarkers. However, troponin-I elevation has a low sensitivity (34%) for myocarditis.
The white blood cell count may range from completely normal to a mild leukocytosis in patients with myocarditis. Viral myocarditis may cause a nonspecific lymphocytic predominance. Eosinophilia may be associated with drug-induced myocarditis or an infiltrative disease like Churg-Strauss. Thrombocytopenia may be present due to the acute viral illness, or from splenic sequestration due to splenomegaly, which may accompany congestive heart failure.
The serum chemistry in patients with suspected myocarditis varies according to the disease burden of the myocardium. Severely affected individuals may have chemistries that correlate with congestive heart failure, including hyponatremia due to sodium dysregulation and reduced kidney function due to poor cardiac output. Elevated liver transaminases (AST and ALT) along with markers of synthetic function (PT/INR, PTT) may be elevated due to hepatic congestion.
Chest radiography may reveal pulmonary vascular congestion and cardiomegaly due to a dilated cardiomyopathy or pneumonia if the myocarditis is caused by mycoplasma.
The role of echocardiography in the diagnosis of myocarditis is nonspecific. Even in severe cases of myocarditis, echocardiography may only identify a dilated cardiomyopathy or, less commonly, hypertrophic or restrictive cardiomyopathies. Regional wall motion abnormalities may mimic changes commonly seen with ischemia.
Although echocardiography has been the traditional imaging modality of choice, it can be normal in less severe cases of myocarditis. Therefore, computed tomography or magnetic resonance imaging may be considered in the diagnosis of myocarditis when echocardiography is nondiagnostic. MRI identifies myocarditis in 30% of patients with chest pain, elevated troponins, and normal coronary arteries. In cases where edema, capillary leak and necrosis can be assessed, cardiac MRI has a sensitivity of 67%, a specificity of 91%, and a diagnostic accuracy of 78%.
|
Endomyocardial biopsy is the gold standard for diagnosing myocarditis. However, the biopsy procedure has obvious risks, can vary by interpreter, lacks prognostic value, and has a low sensitivity due to sampling error. For these reasons, endomyocardial biopsy should be reserved for cases when other infiltrative disorders are in the differential diagnosis, such as cardiac sarcoidosis or giant cell myocarditis. Histologically, an inflammatory cellular infiltrate may be seen with or without myocyte necrosis.
|
The low incidence of myocarditis precludes performing randomized controlled trials with sufficient power to assess therapeutic regimens. The basis of therapy for patients with myocarditis remains largely supportive. The majority of patients with myocarditis will recover fully. For the patients with a stable clinical course, the basis of therapy resides in angiotensin-converting enzme (ACE) inhibitors, beta-blockers, and diuretic therapy. Patients with severe left ventricular (LV) dysfunction can rapidly progress to cardiogenic shock. These patients may require a left ventricular assist device (LVAD) or extracorporeal membrane oxygenation (ECMO) as a bridge to cardiac transplantation.
Patients with myocarditis may have an increased risk of arrhythmias, requiring temporary pacemakers for bradycardia or complete heart block, or antiarrhythmic agents such as amiodarone for ventricular tachyarrhythmias. For patients who present with or have ventricular arrhythmias after the diagnosis of myocarditis, implantable cardiac defibrillators (ICD) may be considered once the acute phase of the illness has resolved. In addition to patients with ventricular tachycardia or fibrillation, those patients with an ejection fraction less than 35% following resolution of the acute phase of myocarditis, should be considered for ICD implantation. It should be noted that patients under consideration for ICD placement should have a life expectancy longer than one year on optimal medical therapy.
While myocarditis is a lymphocytic infiltrative disease, immunosuppressive agents show only marginal beneficial effects. One small trial suggests that patients with human leukocyte antigen expression on biopsy have modest benefit (ejection fraction improvement and New York Heart Association [NYHA] class) from prednisone and azathioprine.
Pericarditis
Acute pericarditis is defined as signs and/or symptoms consistent with pericardial inflammation of less than three weeks’ duration. The true incidence of pericarditis is difficult to quantify since many cases go undiagnosed. Pericarditis may account for up to 1% of cases of ST elevations on ECG, and account for 5% of emergency room visits. Eighty percent to 90% of cases of pericarditis are idiopathic.
Pericarditis can be an acute, subacute, or chronic process. The etiology of acute pericarditis is often idiopathic or viral, though the pericardium can be affected by numerous other disease processes including infectious, neoplastic, autoimmune, and traumatic causes (Table 132-1).
|
Although the pericardium is not required to maintain life, it serves many functions within the thorax. The pericardium is composed of two layers. An inner layer, the visceral pericardium, adheres to the epicardial surface of the heart and consists of a layer of mesothelial cells. The outer layer is an avascular, fibrous parietal layer and consists of elastin fibers embedded within compact layers of collagen. The visceral pericardium reflects back near the great vessels and forms the inner layer of the parietal pericardium. The pericardial space is formed from these two layers and normally contains up to 50 mL of an ultrafiltrate of plasma. In the pericardium, villi and cilia enhance the resorption of fluid. The capacity of the pericardium to accept increased fluid volume depends on the rate of accumulation. A rapid addition of 50 to 200 mL will dramatically increase pressure and affect cardiac function, whereas a very slow accumulation of large amounts of fluid as in myxedema will have little effect on pressure or hemodynamics.
The collagen matrix of the parietal pericardium gives the pericardium its unique mechanical properties. Functionally, the pericardium facilitates contraction and relaxation of the ventricles and atria, allowing pressure changes on one side of the heart to be transmitted to the other side. Additionally, the pericardium’s mechanical properties may determine cardiac output during exercise via the pericardium’s direct limitation of the cardiac filling volumes.
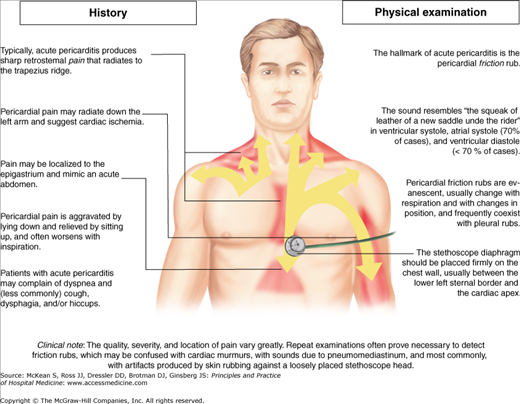
Acute pericarditis presents with chest pain of abrupt onset as the most common complaint. The pain can be quite severe, sharp in character, and nearly always pleuritic in nature. The sharp pain of acute pericarditis differs from the pressure-like discomfort of myocardial ischemia. The pain may be located substernally or located in the anterior chest, and radiation to the neck, arms and shoulders can also occur (Figure 132-1).
The phrenic nerve, which innervates the trapezius muscles, traverses the pericardium; therefore, radiation to the either trapezius ridge of the left shoulder is very specific for pericardial pain. Classically, the pain associated with pericarditis will improve with sitting up and leaning forward and is exacerbated by lying flat or with slight changes in position. Associated symptoms include dyspnea from splinting due to pain, cough, myalgia, arthralgias, and occasionally hiccoughs.
Vital signs in patients with pericarditis may be normal or show low-grade fever and tachycardia. High fever may indicate a bacterial cause, and these patients should undergo echocardiographic evaluation. There may be associated tachypnea from the dyspnea or pain. The most specific, and pathognomonic, finding associated with pericarditis is the pericardial friction rub. This scratchy, superficial sound likely results from inflamed visceral and parietal pericardium rubbing against each other. Classically, there are three components correlating with ventricular systole, ventricular diastole, and atrial systole. All components, two components, one component, or none may be present, as the finding is not sensitive. Two components would be expected in atrial fibrillation due to loss of the atrial component; rapid heart rates may only produce two components due to summation of the early diastolic rub and presystolic rub. The rub may be composed solely of the ventricular component, which is the last to leave over the course of illness. These rubs, described as triphasic, biphasic, or monophasic, may be confused with other cardiac abnormalities, especially in patients with a monophasic rub, which may be mistaken for a murmur.
The pericardial friction rub is best heard at the left sternal border, often in a well-localized spot but may be heard only at the apex. Therefore, the examiner needs to take a systematic approach to auscultation; position the patient forward or even on his hands and knees with the chest down or lying on his abdomen propped up on his elbows; use the diaphragm of the stethoscope against the chest wall with forced expiration; and listen over the entire left precordium. Frequent examinations may be required to hear the friction rub given its fleeting nature. The sound has been described as the sound produced when walking through snow, or pulling apart two pieces of Velcro. The rub of pericarditis may sound very similar to the harsh sound of Hamman crunch (heard in some patients with pneumomediastinum); care should be taken to differentiate these two auscultatory findings. In uncomplicated pericarditis, the jugular venous waveforms remain unchanged.