Chapter 36 Multidrug-Resistant Bacteria
3 What are the risk factors for MDR infections?
Major risk factors for colonization or infection with MDR organisms are as follows:
4 How do mutations in cell wall synthesis contribute to MDR?
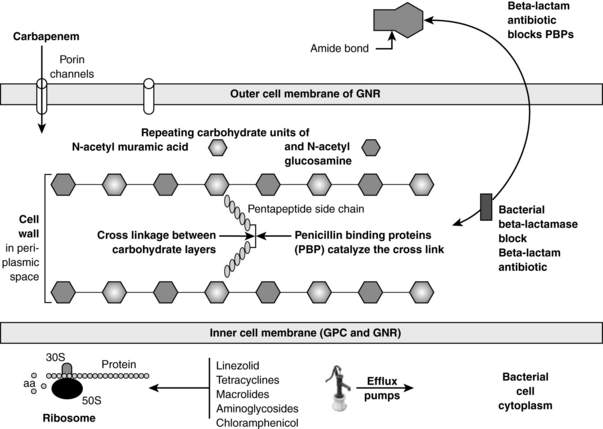
Almost all bacteria have cell walls that are located outside of the inner membrane and are composed of repeating carbohydrate units of N-acetylmuramic acid and N-acetylglucosamine. See Figure 36-1. The key structural stabilizing step is the cross-linking between the carbohydrate layers. Penicillin-binding proteins (PBP) are the bacterial enzymes that accomplish this cross-linkage. β-Lactam antibiotics (penicillins, cephalosporins, carbapenems, and monobactams) bind to PBP, inactivating them, thus interfering with the cross-linkage. Gram-positive bacteria can become MDR by mutations in the PBP such that the β-lactam cannot bind to them. Methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant enterococcus (VRE) all have evolved mutated PBP.
5 Why are β-lactamases so important in causing MDR infections?
β-Lactamases are bacterial enzymes that inactivate β-lactam antibiotics by opening the amide bond of the β-lactam ring. See Figure 36-1. These enzymes are the most common cause of MDR in GNR. The β-lactamases causing the most common MDR in the ICU include the following:
 Extended-spectrum β-lactamases (ESBL) cause resistance to most β-lactam antibiotics with the exceptions of the cephamycins (cefoxitin, cefotetan) and carbapenems. The most common bacteria carrying ESBL are Klebsiella spp. and Escherichia coli. Less commonly Enterobacter, Serratia, Morganella, Proteus, and Pseudomonas aeruginosa spp. may harbor these genes. The genes for these enzymes are carried on chromosomes or plasmids and are thus transferrable between bacteria. ESBL-producing bacteria are also often resistant to aminoglycosides and quinolones. These enzymes are usually inhibited by β-lactamase inhibitors such as clavulanic acid, sulbactam, and tazobactam.
Extended-spectrum β-lactamases (ESBL) cause resistance to most β-lactam antibiotics with the exceptions of the cephamycins (cefoxitin, cefotetan) and carbapenems. The most common bacteria carrying ESBL are Klebsiella spp. and Escherichia coli. Less commonly Enterobacter, Serratia, Morganella, Proteus, and Pseudomonas aeruginosa spp. may harbor these genes. The genes for these enzymes are carried on chromosomes or plasmids and are thus transferrable between bacteria. ESBL-producing bacteria are also often resistant to aminoglycosides and quinolones. These enzymes are usually inhibited by β-lactamase inhibitors such as clavulanic acid, sulbactam, and tazobactam.
 AmpC cephalosporinases are β-lactamases that confer resistance to cephalosporin antibiotics (including cephamycins) by Enterobacter, Nitrobacteria, Morganella, Serratia, and P. aeruginosa. In contrast to ESBL enzymes, they are most often chromosomally encoded and not transferable. Paradoxically they are inducible by third-generation cephalosporins. More recently these genes have been found on transferable plasmids but are not inducible. These enzymes are often resistant to β-lactamase inhibitors.
AmpC cephalosporinases are β-lactamases that confer resistance to cephalosporin antibiotics (including cephamycins) by Enterobacter, Nitrobacteria, Morganella, Serratia, and P. aeruginosa. In contrast to ESBL enzymes, they are most often chromosomally encoded and not transferable. Paradoxically they are inducible by third-generation cephalosporins. More recently these genes have been found on transferable plasmids but are not inducible. These enzymes are often resistant to β-lactamase inhibitors.
 Carbapenemases are enzymes that can inactivate the carbapenems (meropenem, imipenem-cilastatin, ertapenem, and doripenem). These enzymes may also be able to inactivate all classes of β-lactam antibiotics and are resistant to β-lactamase inhibitors. Organisms found to carry these MDR genes include P. aeruginosa, Acinetobacter, Stenotrophomonas, Klebsiella, Serratia, Enterobacter, E. coli, and Citrobacter. In 2009, a new carbapenemase was isolated in pathogens from New Delhi, India, called the New Delhi metallo-β-lactamase-1 (NDM-1). Bacteria harboring the NDM-1 include Klebsiella, E. coli, Enterobacter, Nitrobacteria, Morganella, Providencia, Acinetobacter, and P. aeruginosa. The resistance gene is carried by a plasmid and is thus transferable between bacteria. Isolates have now been found in Europe and the United States. Transmission of aminoglycoside and quinolone resistance may be carried by other genes on the plasmid.
Carbapenemases are enzymes that can inactivate the carbapenems (meropenem, imipenem-cilastatin, ertapenem, and doripenem). These enzymes may also be able to inactivate all classes of β-lactam antibiotics and are resistant to β-lactamase inhibitors. Organisms found to carry these MDR genes include P. aeruginosa, Acinetobacter, Stenotrophomonas, Klebsiella, Serratia, Enterobacter, E. coli, and Citrobacter. In 2009, a new carbapenemase was isolated in pathogens from New Delhi, India, called the New Delhi metallo-β-lactamase-1 (NDM-1). Bacteria harboring the NDM-1 include Klebsiella, E. coli, Enterobacter, Nitrobacteria, Morganella, Providencia, Acinetobacter, and P. aeruginosa. The resistance gene is carried by a plasmid and is thus transferable between bacteria. Isolates have now been found in Europe and the United States. Transmission of aminoglycoside and quinolone resistance may be carried by other genes on the plasmid.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree