CBF
Cerebral blood flow
CMRO2
Cerebral metabolic rate of oxygen
O2ER
Oxygen extraction ratio = (O2 consumption/O2 delivery) = AVDO2 × CBF/CaO2 × CBF = AVDO2/CaO2 = SaO2 − SjvO2/SaO2
SaO2
Arterial oxygen saturation
PaO2
Oxygen tension pressure in arterial blood
SjvO2
Oxygen saturation in the jugular bulb blood
PjvO2
Oxygen tension pressure in jugular venous blood
AVDO2
Arteriovenous difference of oxygen = (SaO2 − SjvO2) × 1.34 × Hb+(PaO2 − PjvO2) × 0.0031
CaO2
Arterial oxygen content = (SaO2 × 1.34 × Hb) + (PaO2 × 0.0031)
CjvO2
Jugular venous oxygen content = (SjvO2 × 1.34 × Hb) + (PjvO2 × 0.0031)
Rationale for SjvO2 Monitoring
The jugular venous oxygenation reflects a balance between cerebral oxygen supply and metabolic consumption , the SjvO2 value being directly proportional to cerebral oxygen supply and inversely proportional to cerebral oxygen consumption. Consequently, SjvO2 monitoring provides a means to indirectly assess the ability of the brain to extract and metabolize oxygen. In the healthy brain, the cerebral metabolic rate for oxygen (CMRO2) is coupled to the CBF such that when CMRO2 increases, CBF increases to match demand. Using the Fick principle, cerebral oxygen consumption can be calculated as:
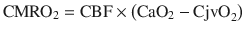 where
where 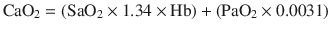 and
and 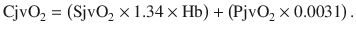
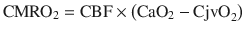
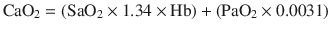 and
and 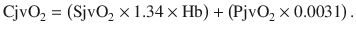
The dissolved oxygen is negligible and can be ignored. Also, the hemoglobin is constant. As a result, the content of oxygen is, in fact, proportional to the oxygen saturation. Hence, the difference in arterial-venous content of blood (AVDO2) can be determined by the difference in SaO2 and SjvO2. Rearranging the above equation,
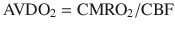 Therefore, SjvO2 is a function of the arterial oxygen saturation (SaO2), CBF, and CMRO2. In a situation in which CMRO2 increases without a concomitant increase in CBF, the AVDO2 increases in conjunction with cerebral oxygen extraction and decreases in SjvO2. As a result, in the clinical situations, a decrease in SjvO2 indicates either relative increase in CMRO2 or a relative decrease in CBF and can help the clinician make a differential diagnosis of cerebral desaturation. Importantly, during disease states such as traumatic brain injury (TBI) as well as under anesthesia, the normal relationship between cerebral oxygen consumption and cerebral blood flow is often altered (uncoupling). However, the clinical value of SjvO2 monitoring lies in the fact that even in such conditions, the oxygen content of jugular venous blood continues to reflect the relative balance between oxygen consumption and supply. Notably, both hypoxia and anemia can reduce the oxygen delivery to the brain.
Therefore, SjvO2 is a function of the arterial oxygen saturation (SaO2), CBF, and CMRO2. In a situation in which CMRO2 increases without a concomitant increase in CBF, the AVDO2 increases in conjunction with cerebral oxygen extraction and decreases in SjvO2. As a result, in the clinical situations, a decrease in SjvO2 indicates either relative increase in CMRO2 or a relative decrease in CBF and can help the clinician make a differential diagnosis of cerebral desaturation. Importantly, during disease states such as traumatic brain injury (TBI) as well as under anesthesia, the normal relationship between cerebral oxygen consumption and cerebral blood flow is often altered (uncoupling). However, the clinical value of SjvO2 monitoring lies in the fact that even in such conditions, the oxygen content of jugular venous blood continues to reflect the relative balance between oxygen consumption and supply. Notably, both hypoxia and anemia can reduce the oxygen delivery to the brain.
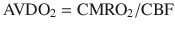
Essentially, the SjvO2 monitoring helps in detecting episodes of cerebral hypoxia/ischemia that may lead to poor neurologic outcome [2]. Based on SjvO2 monitoring, appropriate interventions can be made to either decrease the elevated CMRO2 due to seizures or hyperthermia or to optimize hemodynamic and ventilation parameters to increase the reduced CBF in the setting of below normal SjvO2 values. Conversely, in situations of cerebral hyperemia characterized by supranormal SjvO2 values, depending on the clinical setting, either the CBF may be reduced by blood pressure reduction (e.g., after resection of intracranial arteriovenous malformation) or the anesthetic depth/sedation reduced as appropriate.
Anatomy of Cerebral Venous Drainage
Figure 14.1 shows a diagrammatic representation of cerebral venous drainage. The cerebral venous system is composed of superficial and deep venous plexuses . Venous blood from the brain flows via superficial and deep cerebral veins into the venous sinuses (which lie between the outer endosteal and the inner meningeal layer of the dura mater). Venous sinuses drain into the internal jugular veins (IJVs). The cerebral veins can also be referred to as external (superficial or cortical) and internal (deep) groups. The external group drains mostly into the superior sagittal sinus and, to a lesser extent, to the cavernous or the sphenoparietal sinuses . The internal group of cerebral veins drains into the straight sinus that, together with the superior sagittal sinus, joins to form the confluence of sinuses (torcular Herophili). The two lateral sinuses take origin from the confluence and course laterally to reach sigmoid sinuses and then the jugular bulbs. The jugular veins drain blood predominantly from the ipsilateral side, but also from the contralateral side (minority). Ultimately, all the venous sinuses drain into the IJVs, which comprise two bulbs, the superior and inferior. The superior bulb provides an anatomically viable sampling site. Of the two lateral sinuses, the right is larger in 62 % of the patients, the left is larger in 26 %, and the sinuses are of the same size in 12 % [10]. Cavernous and circular sinuses at the base of the brain have equally free communication from side to side and drain through the petrosal sinuses to the jugular bulbs. Although the dural sinuses join at the confluence of sinuses in most people, the blood from the straight sinus (draining subcortical areas) tends to flow into the left lateral sinus and the blood from the superior sagittal sinus (draining cortical areas) tends to flow into the right lateral sinus [11].
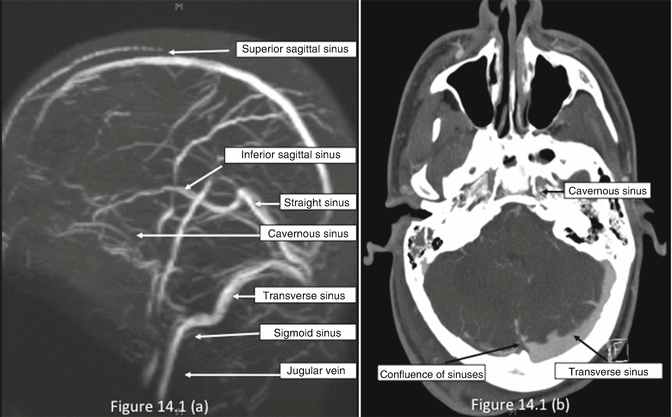

Fig. 14.1
Cerebral venous sinuses : (a) superior view. (b) Oblique posterior view. Note the convergence of several individual sinuses on the internal jugular vein (IJV) near the jugular bulb (Reproduced with permission from the original author of Clinical Anatomy Principals [Chapter 2, page 157] published by Mosby Yearbook Inc. 1996)
On average, two thirds of the blood from a single internal carotid artery is drained by the ipsilateral IJV, and one third is drained by the contralateral jugular vein [12]. Although the jugular venous blood is derived from both cerebral hemispheres, the venous drainage is usually asymmetrical, with the majority of the patients draining cerebral venous blood dominantly into the right side jugular bulb, some draining dominantly into the left side and a few may drain equally into both jugular bulbs. In patients draining primarily into one jugular bulb, a side-to-side asymmetry of greater than 10 % in SjvO2 values can occur [1]. Hence, it is recommended that a jugular bulb catheter be placed into the IJV on the side of dominant cerebral venous drainage [1, 4] although it is often argued that in the presence of a focal brain injury, the catheter should be placed on the side ipsilateral to brain injury. The cerebral venous dominance can be easily determined by examining the venous caliber on the cerebral angiogram (a larger caliber indicating dominant venous drainage) [4], by ultrasonographic comparison of the size of the IJVs [13], or computerized tomographic assessment of the jugular foramen size [14]. Figure 14.2 shows the venous phase of cerebral angiograms of three different patients demonstrating right-dominant, left-dominant, and right-left co-dominance of cerebral venous drainage. In a series of 32 patients with severe TBI and simultaneous measurements of SjvO2 in the right and left jugular bulbs, the average difference in SjvO2 between the right and left jugular bulb was 5 % [15]. Fifteen patients had a maximal right-to-left difference in SjvO2 of more than 15 % [15]. Three additional patients had differences more than 10 % [14]. It was not possible to predict on the basis of CT scan information which patients would have significant differences in SjvO2 or which jugular bulb would have the most abnormal values [15]. These findings further support selecting the jugular bulb on the side of dominant venous drainage for SjvO2 monitoring [4].
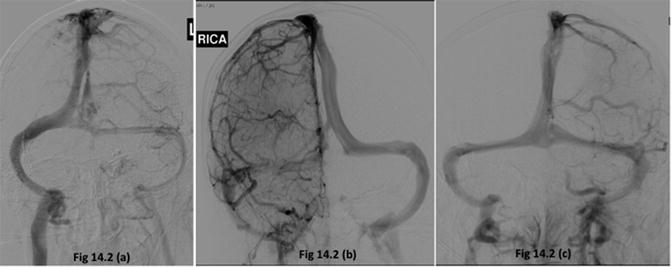

Fig. 14.2
Venous phase of cerebral angiograms of three different patients demonstrating right-dominant (a), left-dominant (b), and right-left co-dominance (c) of cerebral venous drainage
The IJV is a direct continuation of the intracranial sigmoid sinus (see Fig. 14.1). It begins at the jugular foramen of the skull and ends at the thoracic inlet (behind the manubrium sterni where, after crossing the subclavian artery, it joins the subclavian vein to form the brachiocephalic trunk). It thus traverses the entire length of neck, enclosed in the carotid sheath, in close proximity to the carotid arteries. During its course, it lies posterolateral to the carotid artery and crosses it from the lateral to the medial side. The IJV has two dilatations: one at each end, referred to as the superior and inferior “jugular bulbs.” The superior (upper) bulb is an outpouching of the venous wall and is more prominent, whereas the inferior (lower) bulb is a dilation of the vein situated about 1 cm above the clavicle and is less defined.
The previously mentioned, anatomic facts have important clinical implications pertaining to extracranial contamination of blood sampled from the jugular bulb. Such contamination can come from multiple sources: (1) emissary and frontal veins drain blood into the superior sagittal sinus; (2) the sigmoid sinus receives blood from the cavernous sinus through petrosal sinuses. The cavernous sinus in turn communicates with the ophthalmic veins and pterygoid venous plexus; (3) influx of blood from pharyngeal venous plexus and facial veins, which can be a source of contamination if the catheter tip migrates caudally, below the superior bulb. Contamination by extracranial blood will give false high readings of SjvO2 because oxygen extraction by extracranial tissues is less than that of the brain.
Technical Aspects of Jugular Venous Oximetry
Placement of Jugular Bulb Catheter
Under careful aseptic precautions, the IJV on the side to be cannulated is typically punctured under ultrasound guidance at the level of the apex of the triangle formed in the neck by the two heads of the sternocleidomastoid muscle and the clavicle. The Trendelenburg position, often used for central venous cannulation for improved ultrasonographic visualization of the IJV, may not be an option in patients with poor intracranial compliance. The Seldinger technique can be used to thread the jugular bulb catheter over a guide wire. An introducer sheath (5–6 French size) is first inserted through which an oximetric catheter (4.5–5 French) is advanced in a cephalad direction until a resistance is felt (15–20 cm in most adults), which generally coincides with approaching the superior jugular bulb where there is a slight bend in the vessel lumen just below the jugular foramen below the base of the skull. The catheter is then pulled back half a centimeter. The cannulation technique is similar to that for central venous catheterization except that the needle, guide wire, and catheter are advanced in a cephalad direction (Fig. 14.3). We routinely use a 16 G, 5.25-in. long venous cannula for intraoperative jugular venous oximetry [4, 6]. We usually insert the catheter to a distance equal to that measured from the point of insertion to the level of the mastoid process until resistance is met. This technique is helpful in adult as well as pediatric patients [4].
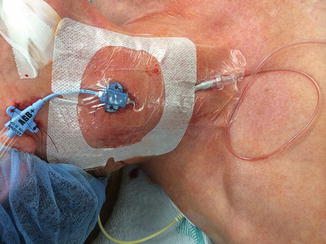

Fig. 14.3
Depicting a central venous catheter and a jugular venous catheter inserted on the same side of the neck. The central venous line is inserted caudad while the jugular bulb catheter is inserted cephalad
The final position of the cathete r in the jugular bulb should be confirmed by a lateral X-ray of the skull documenting the catheter tip at the level of, and just medial to, the mastoid process. It has been suggested that radiographic assessment of jugular venous catheters is best performed using an overpenetrated Stenvers view (with rotation of the head 15°–20° away from the side of the SjvO2 catheter) and a companion over-penetrated lateral radiograph of the neck. Alternatively, on an anteroposterior (AP) view of the skull, a correctly positioned catheter should lie cranial to the line extending from the atlantoaxial joint and below the inferior orbital margin [16]. Accurate positioning of the catheter tip is essential for dependable SjvO2 data. Even when the catheter is placed in a perfect position, it is not unusual for the catheter tip to migrate leading to falsely high SjvO2 values [10]. Hence, the catheter may need readjustment periodically. The presence of an introducer sheath facilitates such adjustments.
It is recommended that the jugular bulb on the side of dominant venous drainage be cannulated. Different suggested approaches to identify the dominant IJV are:
If a cerebral angiogram is available, identify the dominance on the venous phase [4] (see Fig. 14.2)
Use ultrasound to identify the larger caliber IJV, which corroborates with cerebral venous dominance [13]
Cannulate the side with the larger jugular foramen on computerized tomography [14]
Cannulate the IJV, compression of which results in a greater increase in the intracranial pressure (ICP) [17]
In the presence of a focal lesion, cannulate the ipsilateral jugular bulb [18]
Continuous Fiberoptic Jugular Oximetry Versus Intermittent Sampling
Jugular oximetry can be performed either by intermittent sampling of blood or by fiberoptic technology using spectrophotometric catheters that allow continuous displays of SjvO2 values. The underlying principle is based on the differential absorption of light at different wavelengths by oxyhemoglobin and reduced hemoglobin. The Baxter-Edwards system (Edslab Sat II, Baxter Edwards Critical Care Division, Irvine, CA) uses two wavelengths of light for reflectance spectrophotometry and is calibrated against a sample of the patient’s blood. The Abbott system (Opticath Oximetrix, Abbott Critical Care System, Abbott Park, IL) uses three wavelengths of light and may be calibrated in vivo (against the patient’s blood) or in vitro (built-in calibration).
A prospective protocol comparing 195 blood gas measurements with simultaneously recorded continuous bedside oximetric monitor values for 31 ICU patients with TBI undergoing jugular venous oxygenation monitoring for an average of 3.4 days reported acceptable correlation between the in vivo monitor (Baxter-Edwards, Santa Ana, CA) and in vitro co-oximetry [19]. Although the sensitivity of the continuous oximetry was somewhat low (45–50 %), the specificity was 98–100 %, indicating that it is less of a concern that patients may be misdiagnosed as having desaturations resulting in unnecessary interventions [19]. Nonetheless, the investigators recommended that suspected jugular bulb desaturation should be verified before taking therapeutic actions [19]. The fiberoptic catheter has also been noted to provide accurate measures of SjvO2 during neurosurgical procedures in a series of 12 patients with 111 readings ranging from 42 to 95 % [20]. However, in another ICU study of patients with TBI, there was a poor correlation for the first in vivo calibration although subsequently, a close correlation between jugular bulb catheter and oximetry values was demonstrated during episodes of jugular bulb oxygen desaturation resulting from a variety of causes including hypocapnia, hypoperfusion, and intracranial hypertension [21]. These investigators also recommended validation with a laboratory oximeter prior to continuous jugular bulb oximetry [21]. Subsequent to calibration with a laboratory co-oximeter, the Edslab catheter (Baxter Healthcare Corporation, Irvine, CA, USA) was also found to provide SjvO2 values that correlated well with co-oximetry with no appreciable drift and negligible bias [22]. Interestingly, the measurement of SjvO2 by the fiberoptic method compared poorly with bench oximetry during cardiopulmonary bypass (CPB) although there was good agreement between the two methods in the 18 h postoperatively [23]. There were wide limits of agreements (mean difference ± 2 SD) between the two methods during operation (−20.29 to 18.05 %), whereas after operation the limits of agreement were far narrower (−6.39 and 7.45 %) [23]. When indwelling catheters are used for continuous SjvO2 monitoring for several days and/or hemoglobin level is expected to change, it is customary to draw blood samples from the jugular bulb to calibrate the oximeteric catheter at least once daily.
Sampling Rate
Below the jugular bulb, the IJV receives facial and retromandibular venous blood, causing extracranial admixture. Consequently, faster rates of sampling increase the admixture from the extracranial veins, giving falsely elevated SjvO2 values. In mechanically ventilated patients undergoing neurosurgical procedures, Matta and Lam [24] found that sampling at rates faster than 2 mL/min resulted in significantly higher SjvO2 values. Hence, to avoid overestimation of SjvO2, blood samples should be drawn slowly, at approximately 2 mL/min, particularly when the patient is hyperventilated or CBF is otherwise reduced pharmacologically (e.g., during barbiturate therapy) [24]. Importantly, there are no data to prove that the rate of 2 mL/min is free of extracranial blood contamination. Although sampling rates slower than 2 mL/min may further improve accuracy, they may not be practical and may not allow rapid assessment of changes.
Complications
Potential complications are associated with either (1) insertion of the catheters (carotid artery puncture, injury to nerves, injury to lymphatic duct on the left side, pneumothorax) or (2) the presence of an indwelling catheter (infection, thrombosis). In a series of 80 patients, Jakobsen and Enevoldsen [25] had an accidental carotid artery puncture with the Seldinger technique in one patient. Subsequently, Matta et al. [6], in a series of placements of retrograde jugular venous bulb catheters in 100 consecutive anesthetized patients undergoing neurosurgical procedures, reported carotid artery punctures in two patients that were controlled by firm pressure. We have reported our experience of placing jugular bulb catheters in anesthetized pediatric patients and noted no arterial punctures [8]. The use of ultrasound is likely to further reduce the risk of arterial puncture, especially with experienced providers. Serious consequences involving the development of a hematoma that may follow the insertion of a 5–6 French introducer can be avoided, if the smaller catheter (#18g) is always connected to a pressure transducer to ascertain (from the waveform) the presence of the catheter in the vein prior to insertion of the introducer. In a rare case, inadvertent penetration of the posterior wall of the jugular vein during cannulation has been reported, leading to the placement of the catheter tip in the subarachnoid space [26]. Another rare case of unintentional cannulation of the anterior venous plexus of the cervical epidural space during retrograde catheterization of the jugular vein has also been reported [27]. Other complications such as Horner’s syndrome and injuries to the phrenic or recurrent laryngeal nerve may also occur.
The ICP can be increased by obstruction of cerebral venous drainage. Although concerns are sometimes raised about the possible obstruction of cerebral venous outflow by jugular bulb catheters, the size of the catheter used for SjvO2 is very small compared with the size of the vein being cannulated. In fact, Goetting and Preston [28] found no evidence that jugular venous catheterization with indwelling catheters has any significant effect on ICP [28]. Venous thrombosis associated with SjvO2 monitoring has not been reported but could have serious consequences. The risk can be minimized by using the smallest catheter and maintaining patency of the catheter. Infection and sepsis is a known complication with all types of indwelling catheters. Stocchetti et al. [15] reported catheter-induced sepsis in 1.8 % of 45 patients. Strict aseptic technique during insertion and maintenance of catheters is critical.
Contraindications
Absolute contraindications include venous thrombosis. Relative contraindications include hypercoagulable states, coagulopathy, the presence of tracheostomy (due to risk of infection), and unstable cervical spine conditions providing suboptimal patient positioning for placement of the catheter.
Normative SjvO2 Values and Differential Diagnosis of Abnormal SjvO2 Values
The SjvO2 in normal subjects is reported to be in the range of 55–71 % [9]. A later study reported the lower limit of SjvO2 to be lower than previously reported [29]. In humans, confusion develops with hypoxia or orthostatic hypotension when CjvO2 drops from 3 to 2.7 μmol/mL, which corresponds to an SjvO2 of about 45 % [30]. Unconsciousness develops when SjvO2 reaches a value of less than 24 %. In patients undergoing CPB [31] and in patients with traumatic intracranial hematomas [32], increased anaerobic cerebral metabolism was observed when CjvO2 decreased below 3 μmol/mL. This value of CjvO2 corresponds to an SjvO2 of 50 %. In the first 5–10 days after TBI, the SjvO2 is higher, averaging 68.1 ± 9.7 % (range, 32–96 %) [10]. In general, an SjvO2 less than 50 % indicates cerebral ischemia and an SjvO2 greater than 75 % indicates cerebral ischemia.
Figure 14.4 presents a suggested approach to the management of low SjvO2. Briefly, before any intervention, the low SjvO2 value should be confirmed by a laboratory co-oximetry. Low SjvO2 can result from (1) increased cerebral metabolic activity (due to seizures or fever), or (2) decreased oxygen availability (due to hypoxia, anemia, excessive hyperventilation, hypotension, intracranial hypertension, or vasospasm). The decrease in SjvO2 associated with cerebral ischemia is nonlinear such that a minimal decrease in SjvO2 is seen initially when CBF is near normal, becoming more steep as CBF drops below 35–40 mL/100 g/min. In normal humans, the SjvO2 has been noted to remain unchanged with normovolemic hemodilution decreasing the hematocrit to 26 % [33]. Following brain injury, the lower limit of autoregulation of CBF is shifted to the right, resulting in marked decreases in CBF with relatively minor changes in blood pressure or cerebral perfusion pressure [4]. In anesthetized individuals, the effects of anesthetic agents on CBF also need to be considered in the differential. For example, propofol anesthesia maintains the coupling between CBF and metabolism but in conjunction with hyperventilation can cause jugular venous desaturation [34]. Conversely, volatile anesthetic agents cause flow-metabolism “uncoupling” leading to an increase in SjvO2 [35].
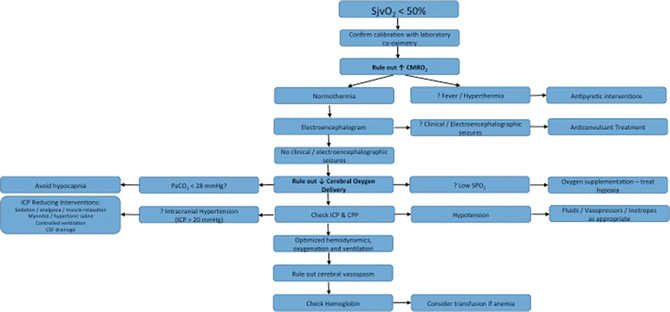

Fig. 14.4
A suggested approach to the management of low SjvO2 value
Cerebral hyperemia (SjvO2 > 75 %) can occur due to intracranial arteriovenous malformations or because of therapeutic hypothermia /sedation to produce burst suppression. High SjvO2 may be treated with controlled moderate hyperventilation or hemodynamic control. Importantly, however, the SjvO2 may be elevated due to cerebral infarction. The therapeutic interventions in response to abnormal SjvO2 values should be made considering information obtained from other monitors simultaneously.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








