Monitoring
Mitchell Donner
William Schoenfeld
I. STANDARD MONITORING
The ASA Standards for Basic Anesthetic Monitoring state that an anesthetist should be present and that oxygenation, ventilation, circulation, and temperature should be continually evaluated during all anesthetics.
A. Standard Monitoring for General Anesthesia
For all anesthetics, the monitoring of circulation, oxygenation, ventilation temperature is required. The minimal requirements for general anesthesia include an oxygen analyzer to confirm the administered FIO2 pulse oximetry, electrocardiogram (ECG), blood pressure measurement, and the ability to assess temperature. Capnography (Chapters 14 and 15) is mandatory for general anesthesia and recommended for monitored anesthesia care and regional anesthesia.
B. Additional Monitoring
The patient’s comorbidities and operative procedure may require additional monitors to measure arterial and venous pressures, cardiac function (Chapters 2 and 24), neuromuscular blockade (Chapter 13), and central nervous system activity (Chapters 11 and 25).
II. CARDIOVASCULAR SYSTEM
Circulation may be assessed with clinical signs, ECG, noninvasive and invasive blood pressure monitoring, central venous pressure (CVP), pulmonary artery cannulation, and echocardiography.
A. Clinical signs and symptoms of perfusion abnormalities are often limited during general anesthetics but are important to assess in the preoperative period (Chapter 1) and may include alterations in mental status, neurologic deficits, dyspnea, chest pain, cool limbs, diminished pulses, and poor capillary refill.
B. ECG. The ECG is used to determine the heart rate and to detect and diagnose dysrhythmias, myocardial ischemia, pacemaker function, and electrolyte abnormalities. The presence of an ECG signal does not guarantee cardiac contraction and output.
1. Mechanism of monitoring
a. Electrode pads. ECG electrodes measure a small electrical signal (about 1 mV). This makes the ECG prone to electrical interference from outside sources and requires proper electrode application to clean, dry skin.
b. Electrode locations. To effectively detect dysrhythmias and ischemia, the pads must be placed in consistent locations on the body. Limb leads must be placed on or near their appropriate limbs and the precordial lead (V5) at the fifth intercostal space, anterior axillary line.
c. Modes and options
1. Monitors often have several choices for filtering of noise, most commonly called “diagnostic” and “monitor” modes. The monitor mode filters out noise by using a narrowed bandpass (0.5 to 40 Hz), while the diagnostic mode filters less signal and noise by using a wider bandpass (0.05 to 100 Hz). The diagnostic mode should be used when monitoring for ischemia.
2. Automatic trending of ST segment changes is useful monitoring for the development of ischemia over time.
2. Rhythm detection. The relationship between the P and QRS waves allows for dysrhythmia diagnosis; the P wave is best seen in lead II.
3. Ischemia detection. Monitoring leads II and V5 allows for detection of ischemia in 95% of patients since it monitors a large area of the myocardium. Lead II monitors the inferior portion of the heart, supplied by the right coronary artery. Lead V5 monitors the bulk of the left ventricle, supplied by the left anterior descending artery. Lead I may be monitored on patients in whom the left circumflex artery is at risk.
C. Arterial Blood Pressure Arterial blood pressure is composed of vascular resistance and blood flow. Blood supply to an organ may be low despite adequate blood pressure because of high vascular resistance. Autoregulation in an individual organ may cause local changes in resistance in order to maintain a constant blood flow.
1. Mean arterial pressure (MAP) may be measured directly or calculated (MAP = diastolic pressure + 1/3 pulse pressure [systolic – diastolic]).
2. Automated noninvasive blood pressure is the most common noninvasive method of measuring blood pressure in the operating room. Manual blood pressure directly measures the systolic and diastolic blood pressures by auscultation of Korotkoff sounds, palpation, or Doppler.
a. Limitations
1. An appropriate cuff size is required for correct determinations of blood pressure. A cuff that is too small may result in falsely high blood pressures while a cuff that is too large may result in falsely low blood pressures. The cuff width should cover two thirds of the upper arm or thigh.
2. Dysrhythmias and motion artifact may result in erroneous values or may not give any values at all, resulting in a delay in accurate measurements when using an automated cuff.
3. Venous congestion and ischemia may result from frequent blood pressure measurements during rapid or large blood pressure fluctuations.
4. Very low or high blood pressures may not correlate with intraarterial measurements; noninvasive blood pressure measurement often overestimates low blood pressure (i.e., systolic blood pressure below 80 mm Hg).
3. Palpation may be used to estimate systolic blood pressure based on whether the pulse may be palpated at key points: radial artery (80 mm Hg), femoral artery (60 mm Hg), or carotid artery (50 mm Hg). This method is inaccurate and is only an estimation when the blood pressure is very low.
4. Invasive blood pressure monitoring uses an indwelling arterial catheter coupled through fluid-filled tubing to a pressure transducer. The transducer converts pressure into an electrical signal to be displayed.
a. Indications
1. Need for tight blood pressure control (e.g., induced hypertension or hypotension).
2. Hemodynamically unstable patient.
3. Frequent arterial blood sampling.
4. Inability to utilize noninvasive blood pressure measurements.
b. Interpretation
1. Systolic blood pressure is often monitored in situations when high pressure may cause rupture (e.g., aneurysm).
2. MAP is often monitored for assessing adequate perfusion pressure of vital organs.
c. Materials include appropriately sized arterial catheter and transducer apparatus. Generally, the catheter size is 22 to 24 gauge for infants, 20 to 22 gauge for children, and 18 to 20 gauge for adults.
1. Transducer is connected to fluid-filled tubing and a pressurized bag of saline. The pressurized saline enables a continuous infusion at 3 mL/hour to prevent clotting. The signal should have a flat frequency response below 20 Hz to monitor all physiologic heart rates.
2. Tubing should be rigid and as short as possible, with no kinks or air bubbles.
3. Setup. The transducer should be electronically zeroed while open to air and placed at the height of the coronary sinus for most patients (phlebostatic axis). Exceptions include placing the transducer at the level of the head during cerebral aneurysm surgery.
5. Procedure: Arterial cannulation
a. Locations. The radial artery is the most common site of insertion. Other locations include ulnar, brachial, axillary, femoral, and dorsalis pedis arteries. As distance from the heart increases, systolic blood pressure increases, diastolic pressure decreases, and MAP generally has little variation.
b. Procedure: Radial artery cannulation. (https://www.youtube.com/watch?v=7coTBnJt4iA)
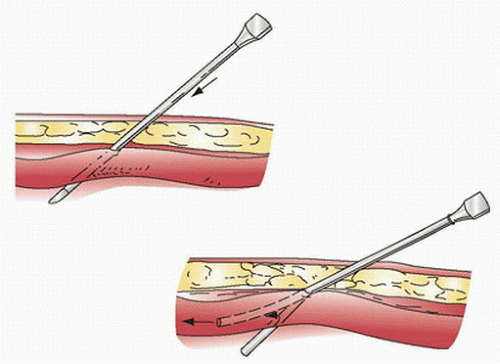
1. The wrist is hyperextended with an arm board and the skin prepped. The procedure should be performed aseptically. Local anesthetic may be used to raise a skin wheal if the patient is awake (Fig. 10.1).
2. The needle is advanced until the artery is entered, and blood flow is observed. The catheter is then advanced over the needle into the artery as in inserting an intravenous catheter. The needle is removed once the catheter is in the vessel, and the catheter is connected to the transducer tubing. An alternative method (transarterial) is when the arterial catheter enters the artery confirmed by a small flash of arterial blood and then advanced through the back wall of the artery. The needle is removed, the catheter is slowly withdrawn, and when there is a spurt of arterial blood, a sterile guide wire is used to ease catheter insertion.
3. Ultrasound guidance is helpful for arterial line placement in patients with poor arterial access.
4. Do not flush the line with more than 3 mL since retrograde flow into the cerebral circulation has been demonstrated.
c. Considerations for Placement
1. Femoral and axillary artery cannulation is best performed with an 18- or 20-gauge catheter to enter the vessel and then inserting a longer 6-inch 18-gauge catheter via the Seldinger technique.
2. The modified Allen test may assess the relative contribution of the radial and ulnar arteries to the blood supply to the hand, but the results are unreliable.
3. Blood pressure and pulse should be assessed in both right and left sides; if disparity exists, the catheter should be placed on the side with the higher pressure since pressure artifacts tend to underestimate the correct blood pressure.
4. Prior cannulation may result in thrombosis. Proximal pulsation should be assessed before placement. Distal pulsation may simply indicate collateral flow.
d. Complications
1. An overdamped waveform will cause an artifactual low blood pressure measurement. This may result from arterial obstruction, catheter occlusion, excess tubing, stopcocks, air bubbles, or kinking of the pressure tubing.
2. An underdamped waveform will result in an artifactually high systolic blood pressure measurement. This may result from use of nonrigid tubing or hyperresonance caused by reverberation of pressure waves.
3. Rare complications include arterial thrombosis, ischemia, infection, and fistula or aneurysm formation. Pulse oximetry on the same hand as the arterial catheter may help to indicate impending vascular compromise. The catheter should be removed, and the opposite side used if new placement is indicated. The ipsilateral ulnar artery should not be cannulated in the event of radial artery complications.
III. CVP AND CARDIAC OUTPUT
A. CVP. CVP is measured by coupling the intravascular space to a pressure transducer using a fluid-filled tubing.
1. Pressure is monitored at the level of the vena cava or the right atrium. The transducer apparatus (see section II.C.4.c.) is placed at the level of the coronary sinus.
a. Indications
1. Measurement of right heart filling pressures to assess intravascular volume and right heart function
2. Drug administration to the central circulation
3. Intravenous access for patients with poor peripheral access
4. Indicator injection for cardiac output determination
5. Access for insertion of pulmonary artery catheter (PAC)
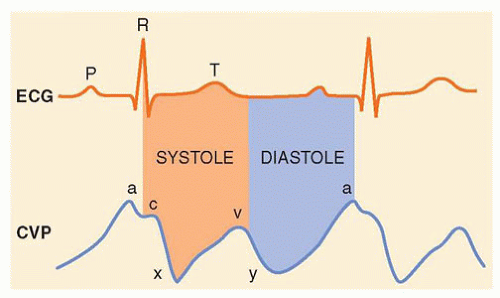
b. Waveform. The CVP tracing contains three positive deflections—the a, c, and v waves—and two negative slopes—the x and y descents (Fig. 10.2). The waves correspond to atrial contraction, isovolemic ventricular contraction including tricuspid bulging, and right atrial filling, respectively. The x descent corresponds to atrial relaxation and systolic collapse. The y descent corresponds to early ventricular filling and diastolic collapse.
c. Analysis
1. Range. The CVP is read between the a and c waves at end-expiration, thus minimizing the interaction of respiration. The normal CVP is 2 to 6 mm Hg.
2. Decreases in CVP. When a CVP decrease is associated with an increase in blood pressure, without changes to the systemic vascular resistance, the CVP has fallen because of increased cardiac performance. If blood pressure is decreased, decreased CVP is due to decreased intravascular volume or venous return.
3. Increases in CVP. When this increase is associated with increased blood pressure, without changes to the systemic vascular resistance, the cause of increased CVP is an increase in volume or venous return. With an associated decrease in blood pressure, the increased CVP is due to decreased cardiac performance.
d. Pathology and CVP
1. Cannon a waves are caused by the atrium contracting against a closed tricuspid valve, as during atrioventricular dissociation.
2. Large v waves are caused by regurgitant flow during ventricular contraction, as with tricuspid regurgitation.
e. Positive-pressure ventilation affects both cardiac output and venous return. According to the Starling rule, the transmural pressure, which is the difference between the atrial pressure and extracardiac pressure, correlates with the cardiac output. At low levels of PEEP,
the CVP increases with increased PEEP. At high levels of PEEP (over about 15 cm H2O), CVP increases as the cardiac output is depressed because of impaired right ventricular output.
the CVP increases with increased PEEP. At high levels of PEEP (over about 15 cm H2O), CVP increases as the cardiac output is depressed because of impaired right ventricular output.
2. Procedure: CVP
a. Locations. Common locations include the following veins: internal jugular (IJ), subclavian, external jugular, axillary, cephalic, and femoral.
b. Materials include a saline bag under pressure, fluid-filled tubing, and transducer. The transducer is placed at the level of the coronary sinus.
1. Multiple lumen catheters are directly inserted and are available with one to four lumens to provide access for multiple drugs, pressure monitoring, and blood sampling.
2. An introducer is a large-bore catheter with a septum valve. A special multiple lumen catheter or a PAC is then placed through the introducer as described below.
3. Ultrasound can be used to identify anatomy, assist catheter insertion, and verify placement.
c. Complications
1. Dysrhythmias, caused by the guide wire irritating the endocardium, are temporary and resolve with withdrawal of the wire.
2. Arterial puncture can cause significant vessel damage and bleeding if the dilator or catheter is placed into the artery. Before dilation, intravenous position should be verified by color, blood gas, or pressure measurement through the finder needle, thin-walled needle, or 18-gauge catheter. If an artery is punctured before dilatation, the needle should be removed and pressure applied for at least 5 minutes (10 minutes in the case of coagulopathy) and a new site chosen. If the catheter is placed in the artery, it should remain in place and a vascular surgeon should be consulted.
3. The guide wire should not feel tethered with dilator placement, as this may signify venous damage or puncture of the posterior wall. Do not continue to advance a guide wire if it does not pass easily.
4. Pneumothorax, hemothorax, hydrothorax, chylothorax, or pericardial tamponade may become evident with vital sign changes. They are in part ruled out with chest radiography. The risk of pneumothorax is highest with subclavian vein insertion.
5. Infection and air embolism may occur at any time before removal of the catheter. The risk of infection is higher with femoral venous placement. To reduce the chance of air embolism upon catheter removal, the site is occluded with the patient performing a Valsalva maneuver. The Trendelenburg position helps to prevent air entrainment at neck and subclavian sites.
d. For the internal jugular Seldinger technique, the right side is preferred because the vessels run a straighter course to the right atrium (Fig. 10.4). (https://www.youtube.com/watch?v=KSgw1V4bchM; https://www.youtube.com/watch?v=2VYp0rEr_cE)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree