Mitral Regurgitation
Phoebe A. Ashley
Carlos A. Roldan
Mitral regurgitation (MR) is the most common acquired valve disease in adults.
MR caused by an anatomic abnormality of the leaflets and/or chordae tendineae is termed primary or organic MR.
MR caused by disruption of the normal mitral apparatus due to dilatation or systolic global or regional dysfunction of the left ventricle (LV) is termed secondary or functional MR.
The history and physical examination continue to be the method of choice for the initial assessment of patients with suspected MR.
The history and physical examination, however, have several limitations:
Patients with severe MR may develop asymptomatic LV dilatation or systolic dysfunction.
The audibility of mild MR is <20%, and that of moderate MR is uncertain.
Patients with moderate to severe systolic heart failure and severe MR frequently have a soft, even inaudible, murmur.
In acute severe MR, the systolic murmur is frequently inaudible.
Eccentric MR jets may produce misleading physical findings. In posterior mitral leaflet prolapse, the mid to late systolic murmur radiates anteriorly and can be mistaken for aortic stenosis.
The physical examination is limited in defining the etiology and severity of MR and of associated LV dilatation and dysfunction.
Common Etiologies
Chronic Mitral Regurgitation
Mitral valve prolapse (MVP) with or without chordal rupture or flail mitral leaflet is the most common cause of MR in the United States.
Ischemic heart disease with global or regional LV systolic dysfunction with or without associated papillary muscle dysfunction.
Rheumatic heart disease.
Acute Mitral Regurgitation
Primary chordal rupture is the most common cause of severe acute MR.
Ischemic papillary muscle dysfunction with or without partial or complete papillary muscle rupture.
Infective endocarditis (IE).
Traumatic chordal or papillary muscle rupture.
Mechanisms of Mitral Regurgitation
Carpentier Mechanisms of Mitral Regurgitation
Type I: Normal leaflet motion with malcoaptation (dilated cardiomyopathy).
Type II: Excessive leaflet motion often due to leaflet prolapse (myxomatous degeneration with chordal elongation or chordal or papillary muscle rupture).
Type III: Restricted leaflet motion:
IIIa: Diastolic restriction (rheumatic type).
IIIb: Systolic restriction (ischemic type).
Echocardiography
Class I or Appropriate (Score 7–9) Indications for Echocardiography
In patients with MR, two-dimensional (2D) and real time three-dimensional (RT3D) transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) play an essential role in determining the
etiology, severity, impact on LV size and function, and timing of valve replacement or repair (2,3) (Table 6.1).
Table 6.1 Class I or appropriate (score 7–9) indications for echocardiography in patients with mitral regurgitation
Baseline TTE evaluation to diagnose and quantify the hemodynamic severity of MR, assessment of leaflet morphology, LV size and function, LA size, pulmonary arterial pressures, and RV size and function in any patient with clinically suspected MR.
TTE/TEE for delineation of the mechanism of MR.
TTE for re-evaluation of the severity of MR, mitral valve apparatus, LV function, and hemodynamics after a change in signs and symptoms.
TTE annual surveillance of LV function (estimated by ejection fraction and end-systolic dimension) and pulmonary artery pressure in asymptomatic patients with moderate or severe MR.
TTE for assessment of changes in hemodynamic severity and ventricular compensation in patients with known MR during pregnancy.
TEE for evaluation of MR in patients in whom TTE provides nondiagnostic images regarding severity or mechanism of MR and/or status of LV function.
TEE preoperatively and/or intraoperatively to establish the anatomic basis for severe MR and to assess feasibility and guidance of repair.
TTE for evaluation of LV size and function and mitral valve hemodynamics after mitral valve replacement or repair to establish a baseline status.
TTE, transthoracic echocardiography; MR, mitral regurgitation; LV, left ventricle; RV, right ventricle; TEE, transesophageal echocardiography.
All indications with Level of Evidence B or C.
(Adapted from Nishimura RA, Carabello BA, Faxon DP, et al. 2008 focused update into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation. 2008;118: e523–e661; Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/HFSA/HRS/ASNC/SCAI/SCCM/SCCT/SCMR 2011 Appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57:1126–1166.)
M-Mode, Two-dimensional, and Three-dimensional Echocardiography: Mitral Valve Morphology
Best Imaging Planes
TTE: Parasternal long- and short-axis and apical four- and two-chamber views.
Table 6.2 Diagnostic value of three-dimensional transthoracic and transesophageal echocardiography
Definition and localization of mitral leaflet lesions in MVP, endocarditis, and congenital mitral abnormalities.
Accurate identification of individual segment/scallop prolapse or flail to better guide and post-operatively assess surgical valve repair/replacement.
Identification of anatomic and mechanical changes in the mitral annulus that contribute to MR.
Insight into the complexity of the mitral valve structure and valve interrelationships with chordae, papillary muscles, and myocardial walls.
More accurate quantification of regurgitant color Doppler flow parameters.
Improved definition of eccentric MR jets aiding volume quantification.
Improved assessment of the mechanism, location, extent, and severity of paraprosthetic regurgitation due to sewing or annuloplasty ring dehiscence.
Volumetric analysis providing an external view of the heart with multiple internal perspectives.
MVP, mitral valve prolapse; MR, mitral regurgitation.
TEE: Transgastric short- and long-axis and midesophageal four-chamber, two-chamber, and long-axis views.
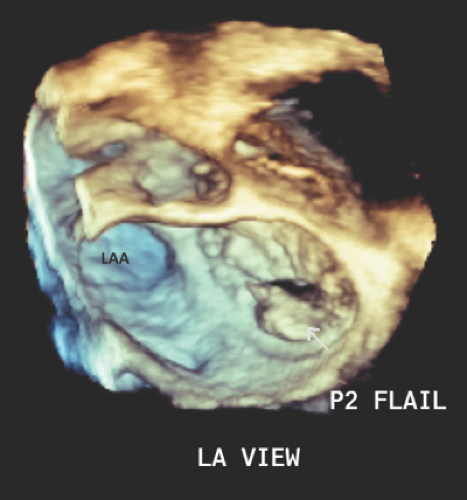
RT3D: Sagittal plane (vertical long axis), coronal plane (four chamber), and transverse plane (short axis). The aorta and left atrium (LA) appendage serve as landmarks for orientation (Table 6.3).
Key Diagnostic Features of Specific Morphology Patterns
Myxomatous Mitral Valve Disease
MVP is the sine qua non of this valve disease.
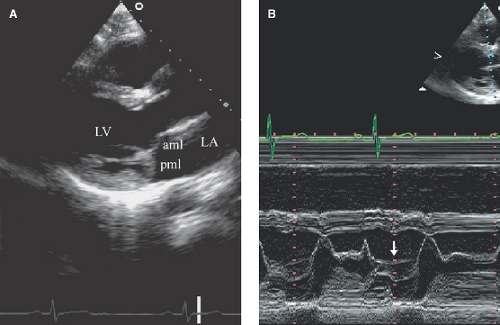
MVP is defined as mid to late systolic or occasionally holosystolic posterior displacement (≥2 mm) of the leaflets beyond the annulus plane (parasternal long-axis view) (Fig. 6.1).
Leaflet thickening (commonly ≥5 mm) (measured during diastasis from long parasternal TTE or during systole from four-chamber TEE views) of soft tissue echoreflectance, redundant chordae, and systolic billowing of the leaflets into the LA.
Leaflet coaptation of prolapsing leaflets may be symmetric or asymmetric (8).
The severity of disease varies from mild displacement of the leaflets to complete prolapse or flail of a portion of the leaflet into the LA.
Table 6.3 Two-dimesional and real-time three-dimensional transesophageal echocardiography of the mitral valve scallops
Cross-sectional Views
Mitral Valve Features Best Identified
2D transgastric basal short axis (0)
C1-C2
2D midesophageal five-chamber
A1/A2-P2
view (0)
2D four-chamber view (0)
A2/A3-P2/P3
2D four-chamber view (40)
A2/A3-P1
2D commissural view (60)
P3-A2-P1 (LAA visible)
2D two-chamber view (90)
P3-A1
2D long-axis view (120–150)
P2-A2
RT3D LA view obtained from midesophageal four chamber view
All 6 scallops, both commisures, entire mitral annulus, and LAA
RT3D LV view obtained from midesophageal four-chamber view
All 6 scallops, both commisures, chordae tendinae, and LVOT
C1, anterolateral commissure; C2, posteromedial commissure; A1,A2,A3 anterolateral, middle anterior, and anteromedial scallops of the anterior mitral leaflet, respectively; P1,P2,P3 posterolateral, middle posterior, and posteromedial scallops of the posterior mitral leaflet, respectively; LAA, left atrial appendage; LVOT, left ventricular outflow tract.
The LAA and aorta serve as landmarks for orientation: C1 and P1 segments are displayed next to the LAA, and A2 is found next to the aorta.
(Adapted from Müller S, Müller L, Laufer G, et al. Comparison of three-dimensional imaging to transesophageal echocardiography for preoperative evaluation in mitral valve prolapse. Am J Cardiol. 2006;98:243–248.)
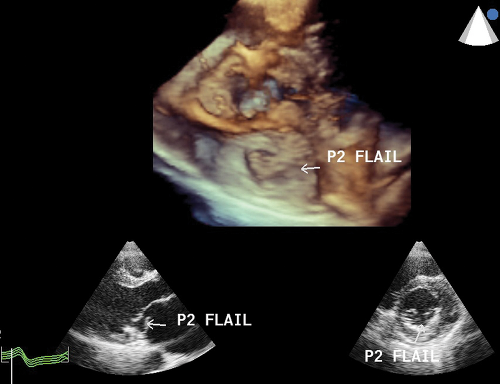
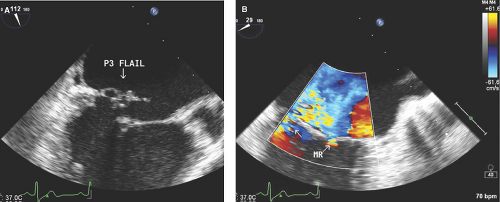
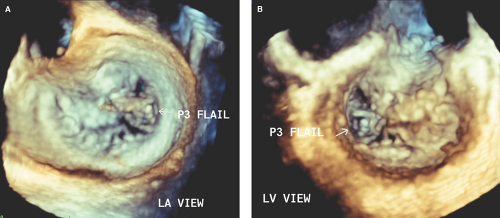
Flail Mitral Leaflet or Chordal Rupture
During systole, the leaflet tip points and extends freely into the LA (Fig. 6.2A,B). The leaflet tip(s) point(s) toward the LV in severe MVP without a flail portion (Fig. 6.1A).
There is noncoaptation or separation of the anterior and posterior mitral leaflets (Fig. 6.2).
There is systolic fluttering of the leaflet in the LA.
Diastolic chaotic motion of the leaflet is frequently seen.
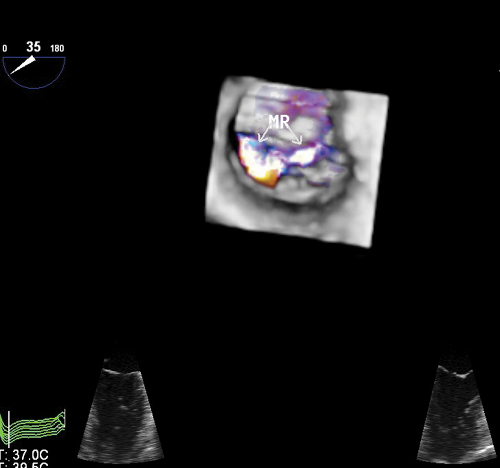
It is always associated with severe and eccentric MR (Fig. 6.2C,D).
Ischemic Mitral Regurgitation
Ischemic MR occurs post myocardial infarction (MI) in the absence of primary valve pathology; the incidence is 20%, and similar for anterior and inferior MI.
In inferior or inferolateral MI, akinesis or dyskinesis of the inferoposterior or inferolateral wall associated with thinning, fibrosis, and/or calcification of the ischemic or infarcted posteromedial papillary muscle causes tethering and decreased mobility of the posterior half of both anterior and predominantly the P2 and P3 scallops of the posterior mitral leaflet. The significantly decreased mobility of the posterior leaflet in relation to the anterior leaflet leads to leaflet malcoaptation and relative anterior leaflet prolapse or pseudoprolapse (10,11,12,13):
The anterior mitral leaflet pseudoprolapse explains the eccentric and posterolaterally directed MR jet.
In anterior MI, LV dilatation and dysfunction lead to downward and lateral displacement of both papillary muscles and downward tethering of the mitral leaflets, leading to incomplete but symmetric malcoaptation and, therefore, a central MR jet.
In chronic ischemic disease, the mechanism of MR is local LV remodeling (apical and posterior displacement of papillary muscles), which leads to valvular tenting toward the LV and loss of systolic annular contraction (4,10,11,12,13).
RT3D TTE or TEE provides further insight into the differentiation of functional and ischemic MR by better delineating derangement of the normal spatial relationships of the mitral valve apparatus (11,12,14) (Figs. 6.10 through 6.13).
By RT3D, the extent of posteromedial mitral leaflets tenting is the predominant determinant in posteroinferior MI and mitral annular flattening and dilatation is the predominant determinant of the regurgitant orifice area in anterior MI or ischemic cardiomyopathy.
RT3D echo has also helped delineate better the frequently asymetric geometry of the mitral annulus (MA) in ischemic MR (Figs. 6.12 and 6.13).
Mixed Myxomatous and Ischemic Mitral Valve Disease
A patient with underlying myxomatous mitral valve disease or MVP may develop coronary artery disease or have an MI, which leads to a mixed mechanism of MR (myxomatous and ischemic valve disease).
Although 2D imaging can determine a mixed mechanism of MR, 3D TEE is more accurate (Figs. 6.14 through 6.16).
Papillary Muscle Rupture (Partial or Complete)
The detached papillary muscle head swings freely from the LV into the LA.
The portion of mitral leaflet attached to the ruptured papillary muscle is flail and associated with severe and eccentric MR (see Fig. 2.13).
Because of the highly eccentric MR jet, TTE underestimates its severity. Therefore, TEE—and, when available, RT3D—should be promptly performed in these patients.
Rheumatic Heart Disease
Thickening and sclerosis, primarily of the leaflet tips.
The leaflets have a diastolic “hockey stick” appearance or a 90-degree angulation from the chordae to the leaflet tip due to commissural and chordal fusion and retraction (see Figs. 7.1 through 7.7).
Incomplete coaptation of the mitral valve due to leaflet retraction.
Relative immobility of the posterior mitral valve leaflet.
Dilatation of the LA with systolic expansion.
In acute rheumatic fever, discrete small vegetations may be identified:
These nodules measure 1 to 5 mm, have a soft tissue echoreflectance, and are located more commonly at the leaflets’ coaptation point.
Unlike IE, rheumatic vegetations are sessile and may not have independent mobility.
In recurrent rheumatic fever, anterior leaflet prolapse may be seen.
Age-related or Degenerative Disease
Mitral annular calcification (MAC) is the sine qua non of degenerative or senile disease.
MAC appears as an area of increased echogenicity on the LV side near the insertion of the posterior leaflet, which moves parallel and anterior to the LV posterior wall.
MAC may extend to the basal portion of the mitral leaflets, especially the posterior leaflet.
MAC may involve a focal area of the annulus or the entire U-shaped posterior annulus.
Rarely, cystic MAC mimicking a mitral annulus mass can be seen.
MAC increases the rigidity of the annulus and impairs its systolic contraction, which in turn leads to MR.
The authors define MAC as mild when the degree of sclerosis in an anteroposterior cross-sectional view of it is <5 mm, moderate if 5 to 10 mm, and severe if >10 mm.
RT3D echo further identifies changes in the annular shape and morphology of degenerative mitral valve disease (14).
Infective Endocarditis
Vegetations are the sine qua non of IE.
Recently formed vegetations appear as circumscribed, soft tissue echoreflective masses, generally at the leaflet tips, with irregular borders and variable shape; they are more commonly pedunculated, but they can be sessile.
Vegetations have a characteristic independent chaotic or rotary motion, involve the anterior or posterior leaflet, and are located on the atrial side of the leaflets’ coaptation point.
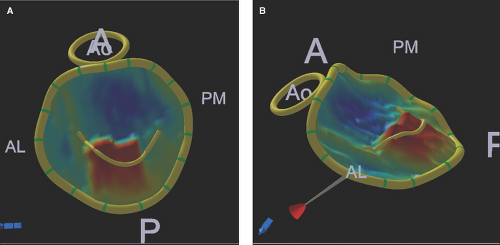
Figure 6.5: A, B. Geometric 3D TEE quantification of the mitral valve and annulus. These 3D TEE LA views (on face A, side B) with geometric quantification of the mitral valve and annulus of the patient discussed in Figure 6.4 illustrate a flail midscallop (in red color) of the posterior mitral leaflet. These 3D geometric reconstructions define accurately that the mitral annulus lost its normal D shape into a circular shape due to enlargement predominantly of the anterior (A) to posterior (P) annular diameters. AL, anterolateral and PM, posteromedial commisures; Ao, aortic valve.
Freely mobile vegetations may prolapse into the LA during systole and may fall into the LV during diastole. Rarely, large vegetations may cause mild valve obstruction.
The underlying leaflets are usually structurally abnormal and have a specific underlying morphology (i.e., myxomatous, rheumatic, or degenerative).
Chronic or healed vegetations have homogeneously increased echoreflectance and are less or non-mobile.
2D and RT3D TEE are superior to TTE for the detection and characterization of vegetations and associated complications (perforation, abscess, pseudoaneurysm, or fistula) (see Figs. 13.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree