Microanatomy of the Peripheral Nervous System and Stimulation Thresholds Inside and Outside the Epineurium
Part 1: Microanatomy
Andre P. Boezaart
Paul E. Bigeleisen
Nizar Moayeri
The use of ultrasound has provided anesthesiologists a deeper undersTanding of the location of peripheral nerves as well as a powerful tool to anesthetize these nerves. The wavelength of medical ultrasound used for nerve blocks ranges from 0.1 to 1 mm. In most cases, the nerves that we wish to image range from 3 to 15 mm in diameter. Under optimal conditions, we may, therefore, be able to “look” inside large nerves or a nerve plexus and “see” individual fascicles. Unfortunately, the resolution provided by ultrasound does not allow us to view the perineurium, a protective sheath that surrounds the fascicles as the nerve root emerges from the lateral recess of the spinal canal and travels toward the periphery as a peripheral nerve. However, in small patients and children, we may be able to image the dura mater where it surrounds the cauda equina and as the nerve root emerges from the lateral recess in the cervical spine. The latter may be of use when performing transforaminal cervical blocks. Some anesthesiologists may not be familiar with the ultrastructure of nerve roots and nerves and, for this reason, may not appreciate the risks involved with paravertebral nerve blocks—even with ultrasound guidance.
Catastrophic outcomes following paravertebral blocks at the cervical,1, 2, 3and 4 thoracic,5 and lumbar levels6, 7and 8 have been reported, some of which were reversible cases of extensive epidural/subdural block or total spinal anesthesia. Unfortunately, other cases resulted in paraplegia, quadriplegia, and death. The explanation for these catastrophes focused on intracord injection, which complicated the blocks performed on patients under general anesthesia. Interscalene block has also been blamed for disastrous outcomes.9, 10, 11, 12, 13and 14 The report by Benumof9 of four cases of spinal injury after interscalene block attracted much attention and generated a fierce debate on the safety of performing blocks on patients under general anesthesia. The conclusions reached by Benumof—that blocks should not be performed under general anesthesia—were, regrettably, largely incorrect, particularly in the case of pediatric patients. These tragic outcomes, like many others, were most likely caused by intraroot injection and had nothing to do with the fact that the patients were under general anesthesia when the blocks were placed. Benumof’s conclusions were largely based on a misundersTanding of the microanatomy of the connective tissue framework of the nervous system. All of these cases have two things in common: root level nerve blocks and thin, relatively sharp needles.15, 16and 17
Our undersTanding of the microanatomy of the peripheral nervous system is already over 130 years old. Key and Retzius18 in 1876 (Richardson’s stain) and Horster and Whitman19 in 1931 (trypan blue) studied the spread of intraneurally injected solutions. In 1948, French et al.20 repeated this work with radiopaque contrast medium in dogs. In 1952, Moore et al.21 used methylene blue-stained exocaine, and in 1978, Selander and Sjostrand22 used radioactive local anesthetic with fluorescent dye to study the microanatomy of the sciatic nerve in rabbits. In the interval, electron microscopy23 was used to confirm what was already known.24
According to conventional teaching, (including many modern anatomy and anesthesia textbooks), the cerebrospinal fluid (CSF) originates in the choroid plexus, is discharged into the cerebral ventricles, and exits through the foramina of Lushka and Magendie. The CSF then gathers in the cisterns at the base of the brain, where it flows to the villi or pacchionian bodies, and then into the peripheral venous circulation.
In 1948, Hassin25 proposed a new depiction of how the CSF courses through the circulation.
In summary:
The CSF is the extracellular fluid of the brain and spinal cord.
The circulation of the CSF also involves the Virchow-Robin spaces that surround the arterioles in the brain; these spaces form the blood-brain barrier.
Absorption of the CSF is not through the villi or pacchionian bodies only but also through the perineurial spaces of the cranial nerves and spinal roots.
The CSF acts as the “lymph fluid” of the central nervous system and carries away waste.
There is no central force, per se, that drives the CSF into the circulation. The cardiac cycle causes expansion and contraction of the brain and spinal cord, which are encased in a rigid compartment. During systole, the entire brain and spinal cord expand, and pressure in the CSF increases. Following a pressure gradient, the CSF flows from the central space out into the perineural spaces of the cranial and spinal nerve roots.
Peripheral nerves and plexuses
The tissue fluid deep to the epineurium, but outside of the perineurium, in a peripheral nerve is lymph and drains to the regional lymph nodes.25 The axons of peripheral nerves are extensions of nerve cells in the central nervous system. These axons, which are surrounded by perineurium, form fascicles and are bathed by CSF. Under normal conditions, the longitudinal flow of the CSF within the fascicle is minimal.22 Lateral extension (centrifugal) of the perineurium is minimal even under high pressure. As the nerve approaches dural penetration, resisTance to lateral extension increases and a peripherally injected medium comes to lie in the clefts of the perineurium. Final emergence into the subarachnoid space appears to occur, first, by way of the subdural space and, subsequently, by breaking through the arachnoid barrier into the subarachnoid space. Injection into peripheral nerve fascicles, which is difficult to achieve under clinical conditions, provides direct access to the CSF and interstitium of the spinal cord. Conversely, penetration and injection into a spinal root is relatively easy under clinical conditions, and this injectate similarly has direct access to the CSF and spinal cord interstitium (Fig. 4.1). The clinical consequence of an injection into a spinal root will depend on the volume, rate, and pressure of the injectate.
Peripheral nerves are composed of numerous fascicles that contain axons. Each fascicle is bounded by a dense perineurium, and a fine epineurial membrane holds the fascicles together (Fig. 4.2). The epineurium consists of a condensation of areolar connective tissue that surrounds the perineural ensheathment of the fascicles on uni- and multifascicular nerves. The attachment of the epineurium to surrounding connective tissue is loose, so that the nerve is relatively mobile except where tethered by entering blood vessels or nerve branches. Greater amounts of connective tissue are normally present where nerves cross over joints, and nerves usually carry sensory signals from branches from those joints. In general, the more fascicles within a nerve, the thicker the epineurium would be. Variable quantities of fat are also present in the epineurium, particularly in larger nerves. This fat cushions the fascicles against injury by compression; thus, large multifascicular nerves are less susceptible to injury by compression than are smaller or unifascicular nerves. The vasa nervorum enter the epineurium, where they communicate with a longitudinal anastomotic network of arterioles and venules. The epineurium also contains lymphatic vessels, which are not present within the fascicles. These lymphatic channels accompany the arteries of the peripheral nerves and pass into the regional lymph nodes.25
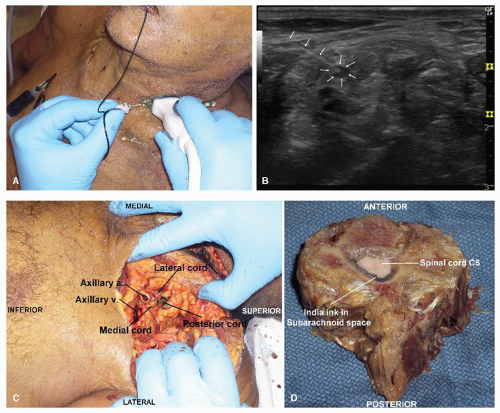 Figure 4.1. A: Ultrasound scan at C5 showing intraneural injection. B: Needle and probe position during injection in A. C:
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|





