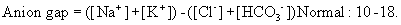Chapter 27 Metabolic disorders
ELECTROLYTE EMERGENCIES
Theory
• Serum electrolyte concentrations are usually tightly controlled unless excessive gains or losses overwhelm homeostatic balance.
• Electrolyte balance requires normal hormonal control of intact kidney function. Primary renal dysfunction or the effects of drugs or endocrine disease can result in abnormalities.
• Water and electrolytes can be mobilised between plasma and other body compartments. This may occur under hormonal control.
Causes/assessment
• Electrolyte losses: renal (drug effects or endocrinopathy), GIT (vomiting, diarrhoea, surgical drains), skin (sweating, burns)
• Electrolyte gains: ↓ renal elimination (renal failure, drug effects, endocrinopathy), GIT (diet, drugs), iatrogenic (intravenous therapy (IVT), total parenteral nutrition (TPN))
Clinical effects
• Electrolytes have a role in establishing the membrane potential, receptor signalling and enzyme function.
• Depending on the electrolyte abnormality the central nervous system (CNS), cardiovascular system (CVS) and/or neuromuscular system can be affected.
Management
Treat the patient, not just the abnormality.
• Severe, life-threatening or resistant cases, e.g. neurological (seizures, coma), CVS (hypotension, abnormal ECG), respiratory muscle failure (hypoventilation, tetany)—get input from the intensive care unit (ICU).
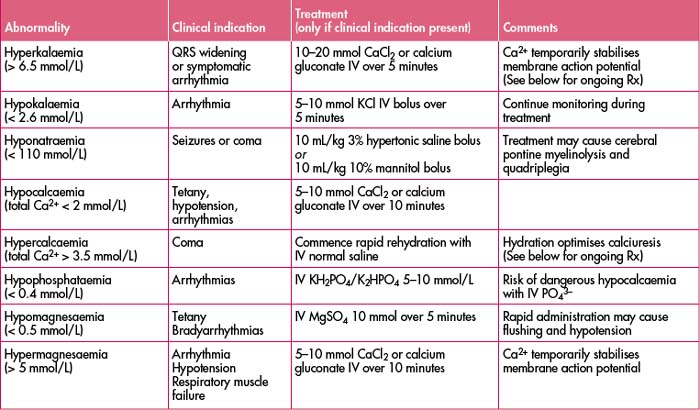
(See Table 27.3 for a list of electrolyte abnormalities, clinical indications and treatment.)
ACID–BASE DISTURBANCES
Pearls/pitfalls
• An acute derangement in acid–base balance invariably signifies a significant disease process (most patients need admission or at least extended observation).
• An abnormal or recent change in venous bicarbonate may be the first clue to an acid–base disturbance.
• Arterial blood gas (ABG) determination is essential to fully categorise the disturbance, e.g. acidosis/alkalosis, respiratory/metabolic or mixed. Don’t just look at pH.
Determining the acid–base abnormality
2. Is the respiratory compensation for a metabolic abnormality appropriate? (See ‘Metabolic equations’ in the ‘Quick reference’ section at the beginning of this book.)
Metabolic acidosis
(Mild: [HCO3–] < 18 mmol/L; moderate: < 15 mmol/L; severe: < 12 mmol/L)
• Important causes of wide anion gap metabolic acidosis: poor tissue perfusion, hypovolaemia and/or sepsis (most common), diabetic ketoacidosis (DKA), renal failure, drug poisoning.
• Less common causes: bicarbonate loss from GUS or GIT/‘normal anion gap metabolic acidosis’ e.g. renal tubular acidosis, surgical fistula/stomal/drain losses, Addison’s disease.
• Rapidly assess the following—circulatory status (HR, BP, urine output), infection (fever), hyperglycaemia (bedside blood sugar level (BSL), urinary ketones).
• Important lab tests—electrolytes, anion gap, renal function, serum osmolarity, serum ketones, BSL, WCC.
Metabolic alkalosis
(Mild: [HCO3–] > 25 mmol/L; moderate: > 30 mmol/L; severe: > 35 mmol/L)
• Common causes in emergency department include: diuretic use, gastric losses (e.g. pyloric stenosis, bulimia nervosa).
Respiratory acidosis
(Mild: pCO2 > 45 mmHg; moderate: > 55 mmHg; severe > 65 mmHg)
• Common emergency department causes include: acute cardiorespiratory illness (asthma/COAD, pulmonary oedema, pneumonia), CNS insults (opiates, benzodiazepines, acute space-occupying lesion), trauma (head injury, flail chest, haemo/pneumothorax).