MESENTERIC ISCHEMIA
CASE SCENARIO
A 59-year-old woman with a past medical history of smoking, hypertension, and claudication was transferred from an outside hospital for further management of acute, diffuse abdominal pain, worst in the right lower quadrant, as well as nausea and vomiting. She was previously seen at the outside institution for similar symptoms, and at that time she was diagnosed with acute cholecystitis and taken to the operating room for laparoscopic cholecystectomy. Intraoperatively, it was found that the gallbladder was not inflamed. After conversion to an open procedure, however, a loop of necrotic jejunum was identified that was resected and primarily reconstructed. The superior mesenteric artery (SMA) was palpated and found to have a normal to slightly diminished pulse. Upon further review of her symptoms, she admitted to approximately 6 months of intermittent, sharp abdominal pain that was most pronounced after eating. Just prior to her acute presentation, the abdominal pain worsened sufficiently to decrease her oral intake.
EPIDEMIOLOGY
The term “mesenteric ischemia” (MI) includes a wide array of diseases that lead to insufficiency of the mesenteric vasculature and subsequent intestinal compromise, often with dire consequences. Acute mesenteric ischemia (AMI) was first recognized as a pathologic entity in 1895, when 2 case reports of bowel resection for compromised mesenteric flow, due to venous as well as arterial thrombosis, were published.1 The fundamental finding of “pain out of proportion to physical examination” was first described in that publication. Furthermore, the acuteness of the disease process and grave prognosis were established in early reports.
Several years later, a related but distinct pathology was defined: chronic mesenteric ischemia (CMI). Brunton was one of the first to report mesenteric vascular insufficiency leading to chronic pain and weight loss.2 He also made reference to the term “angina abdominis” and credited Baccelli with its first use.
In the 1950s, surgeons addressed the cause of mesenteric ischemia and performed thrombendarterectomy of the SMA for chronic disease as well as embolectomy for acute disease.3 The understanding of mesenteric ischemia continued to evolve, as evidenced by descriptions of compressive syndromes such as median arcuate ligament syndrome.4
There are several suggestions for classification of mesenteric vascular insufficiency based on etiology: arterial vs venous, and embolic vs thrombotic. For this chapter we will focus on the radiologic and clinical evaluation of the acute forms of mesenteric ischemia and make some important distinctions about imaging options depending on the acuity of presentation. The acute-on-chronic scenario, with terminal thrombosis of an atherosclerotic SMA stenosis, as presented in the clinical scenario above, is chosen as one of the more likely presentations that general surgeons are called upon to evaluate. It is often insidious in onset, with potential for misdiagnosis and delay in care.
Overall, MI is a rare disease. Within the spectrum of this disease, however, acute presentations are much more prevalent, accounting for about 1 out of 1000 hospital admissions and with an incidence of about 10 per 100,000.5 AMI has a rather balanced male-to-female ratio of 4:5.6 CMI, on the other hand, has an incidence of about 1 to 2 per 100,000, and the male-to-female ratio for CMI has been measured at 1:2.7 The comparative infrequency of CMI may be explained by the fact that many patients with acute atherosclerotic occlusion have chronic, undetected symptoms that predate their acute illness.
For both groups, the usual age of presentation is older than 70 years. The most commonly associated risk factor for either presentation is heart disease. Smoking and hypertension are observed in more than half the patients, and more than a third of the patients have chronic obstructive pulmonary disease.6
PATHOPHYSIOLOGY
Mesenteric ischemia describes the final pathway of mesenteric vascular insufficiency, which progresses to bowel necrosis and, if untreated, death. There are several pathophysiological processes that can lead to mesenteric ischemia. All involve different management strategies and therefore require prompt recognition.
In AMI there are four distinct etiologies. The first is arterial embolism, which accounts for 50% of cases.8 This is usually from a cardiac source, related to atrial fibrillation, myocardial infarction, or valvular heart disease. The SMA is routinely affected due to the anatomic angle and its high flow. The SMA comes off at an angle of approximately 45 degrees and thus allows for easier entry of emboli compared to the celiac artery, which comes off at nearly 90 degrees. Frequently, the embolus lodges in the more distal vessel just beyond the takeoff of the middle colic, sparing the distal right and transverse colon colon and proximal jejunum.
The second etiology is arterial thrombosis, which accounts for 20% of presentations.8 Improved embolic prophylaxis and an increasing prevalence of mesenteric atherosclerosis contribute to a rise in this phenomenon among elderly patients. It is an acute-on-chronic event and requires prior atherosclerosis of the mesenteric vessel origins, which serves as a nidus for thrombosis during a period of low flow, such as dehydration. In many cases classical findings of CMI can be elicited from the history, such as postprandial pain, weight loss, and food fear.
Nonocclusive mesenteric ischemia (NOMI) is the third cause of AMI and accounts for about 20% of cases.7 Similar to arterial embolism and thrombosis, it affects the mesenteric inflow. The main difference, however, is that the inflow vessel is patent. Ischemia occurs when splanchnic blood flow falls below levels required to support basal metabolic demand. This reduction of splanchnic blood flow occurs with all forms of shock, as well as in the setting of prolonged vasoconstrictor use or severe splanchnic vasoconstriction from cocaine or digoxin.9 Patients with NOMI have the worst prognosis due to the high mortality associated with their underlying disease process.
Finally, the least common form of AMI arises from mesenteric venous thrombosis (MVT). It occurs in about 10% of cases and usually affects the superior mesenteric vein (SMV; 70%), followed by the portal and inferior mesenteric veins.10 Thrombosis causes outflow occlusion, which leads to bowel wall edema, impaired microvascular perfusion, and ultimately intestinal infarction. MVT occurs in settings of altered coagulation such as cancer, hypercoagulable syndromes, infections, and severe dehydration.
CMI is best characterized as aortic atherosclerosis that involves the origins of the three mesenteric vessels: the celiac artery (CA) and the superior (SMA) and inferior mesenteric arteries (IMA). As such, it shares the same risk factors as peripheral vascular, cardiovascular, and cerebrovascular disease: smoking, hypertension, hyperlipidemia, diabetes mellitus, and age. These risk factors and comorbidities can be elicited from patients with CMI in the vast majority of cases upon careful questioning.
Despite the 17% prevalence of significant atherosclerotic changes of the mesenteric vessels,11 CMI is rare (incidence 1 in 100,000) and thus difficult to diagnose. The rather vague symptoms and slow development contribute to this difficulty. Patients may remain asymptomatic for a prolonged period due to extensive collateralization between the three mesenteric vessels and the systemic circulation. With CMI, there is multivessel involvement with de facto SMA stenosis or occlusion. Single-vessel CMI is rare except when there is severe SMA disease and collaterals have been interrupted by previous surgery involving bowel resection.
CLINICAL PRESENTATION
Patients with AMI present with acute abdominal pain out of proportion to the physical findings. Specifically, they complain of severe pain but have little tenderness, without guarding or localization. This is especially true in cases of SMA embolus before the onset of intestinal gangrene.
Patients with acute-on-chronic mesenteric ischemia usually present in a more subtle manner, although with the same poorly localized symptoms without focal tenderness. Other suggestive but less specific symptoms are nausea and vomiting, as well as diarrhea, possibly with guaiac positive stool. Frank gastrointestinal bleeding is uncommon.
It is important to perform a focused history to elucidate prior medical problems that might indicate the etiology of AMI and thus guide therapy. Knowledge of recent myocardial infarction, presence of untreated atrial fibrillation, or a prodrome of food fear and weight loss might be helpful. With regard to NOMI, the patient usually presents in shock. Finally, patients with MVT present subacutely and often report symptoms of abdominal pain, anorexia, and diarrhea for 2 to 3 days. MVT can also occur in association with other abdominal pathologies. Pancreatitis, for example, can lead to SMV or splenic vein thrombosis due to hypercoagulability and local inflammation of the retroperitoneum.10
The common pathway of mesenteric ischemia leads to tissue hypoperfusion and bowel necrosis, and the early findings of a soft abdomen without significant tenderness give way to more ominous symptoms. These later findings are absence of bowel sounds and development of guarding and rebound. Laboratory findings include leukocytosis with a left shift, and acidosis with increased lactic acid and anion gap.
Patients with purely CMI, on the other hand, often complain of long-standing symptoms, and may go many months before seeking evaluation. They report postprandial pain that usually starts about 15 to 20 minutes after eating and last for about 3 to 4 hours, the classic “angina abdominis.” They develop food fear and lose 20 to 30 pounds on average before diagnosis. They often report an odyssey from one specialist to another without experiencing improvement in their symptoms. Some are falsely diagnosed with psychiatric diseases. Physical examination is unremarkable, except for cachexia in severe cases. Equally, laboratory findings are normal, although markers of nutrition such as albumin and prealbumin can be low.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of acute and chronic mesenteric ischemia is broad, and delay in diagnosis is common. CMI patients may have many normal studies and even undergo procedures to treat other unrelated problems before the correct diagnosis is made. Even in acute presentations the diagnosis can be unclear.
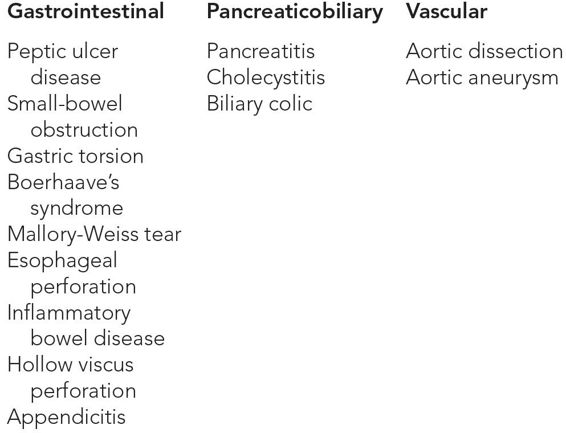
Virtually all intra-abdominal pathologies that cause a rather abrupt onset of pain are in the differential. Among the most common are small-bowel obstruction, biliary tract disease, pancreatitis, volvulus, or complications of inflammatory bowel diseases. When the patient presents with epigastric pain, one has to also think of peptic ulcer disease (especially perforation), gastric torsion, Boerhaave syndrome, Mallory-Weiss tear, and esophageal perforation. Other vascular-related diagnoses include aortic dissection and symptomatic or ruptured aortic aneurysm. Fortunately, the majority of these can be ruled in or out with the same widely available and easily interpretable imaging modality, i.e., computed tomography (CT) scan (Table 13–1).
WORKUP AND CHOICE OF IMAGING
The possibility of rapid decline if appropriate therapy is not instituted requires a fast and ubiquitously available imaging modality to detect mesenteric ischemia. CT fits this description, and is the best choice to diagnose AMI, especially with the development of rapid multidetector CT angiography (CTA).12 Advantages of CTA include its speed, virtually universal availability, and excellent spatial resolution. Axial images and automated 3D reconstructions allow for rapid interpretation of the vascular anatomy out into third-order branches. Atherosclerosis, thrombus, and collaterals are elucidated, together with other nonvascular findings to support the diagnosis of AMI. Disadvantages of CTA include the use of ionizing radiation and the need for a contrast agent, with its risk of allergic reactions and nephrotoxicity.
A meta-analysis of six studies published between 1996 and 2009 on the diagnostic accuracy of multidetector CT (MDCT) in AMI showed a pooled sensitivity of 93% and specificity of 96%.13 The sensitivity and specificity of CTA rely heavily on accurate technique, as well as a complete evaluation of vascular and nonvascular findings to arrive at an overall impression. False positives may be seen with Crohn’s disease and other inflammatory bowel conditions.14
CTA is similarly used to evaluate the atherosclerotic plaque burden of the mesenteric vessels. A normal CTA virtually excludes the diagnosis of mesenteric ischemia. If CMI, however, is present, CTA shows the development of collaterals such as a prominent gastroduodenal artery or a large, “meandering” mesenteric branch from the inferior mesenteric artery (IMA).
The CTA study protocol for evaluation of mesenteric ischemia should include an early arterial, and a late arterial or early venous phase.15 The delayed phase demonstrates the presence or absence of bowel wall enhancement. Fine, overlapping slices (0.5–1.5 mm collimation) should be made through the upper abdomen to minimize volume averaging and to evaluate the celiac axis and SMA with sufficient detail. Traditional “positive” iodine-based oral contrast should not be given, as it tends to detract from image quality because of the artifact created by pooling of high-density material in the GI tract. Instead, a “negative” oral contrast, e.g., water, can be given for enhanced image quality. This allows visualization of early bowel wall enhancement, or lack thereof, during the late arterial phase.
An initial evaluation in the emergency department often includes plain radiographs. They may suggest abdominal pathology, but are neither sensitive nor specific.
Conventional angiography was once the gold-standard diagnostic method, but CTA has replaced it. The role of angiography in the modern treatment of mesenteric ischemia is often more therapeutic than diagnostic. Percutaneous visceral stenting has overtaken open revascularization as the initial treatment for CMI.16 In the acute setting, if a hybrid endovascular operating room is available, angiography may be the final step in both diagnosis and treatment. More often, however, emergent treatment of AMI mandates an open operative approach to assess for and resect nonviable intestine. A variety of revascularization techniques, including bypass, limited thrombolysis, angioplasty, and percutaneous or open retrograde stenting17 of mesenteric vessels, are possible applications or adjuncts.
Magnetic resonance angiography (MRA) offers a second modality for obtaining anatomic imaging of the visceral vessels, aside from CTA. There are, however, important differences. This technology avoids ionizing radiation and iodinated contrast agents. On the other hand, disadvantages include the long time to perform a study, the need for extensive post-processing, and the reduced degree of spatial resolution compared to CTA. It also has reduced visualization of the IMA, peripheral mesenteric vessels, calcified plaques, and previously placed stents compared with CTA.
CMI is not immediately life threatening and thus can be worked up with fewer time constraints and with less invasive testing. Duplex ultrasonography (DUS) is the modality of choice to screen patients suspected of having CMI. It has been validated for more than 30 years,18 and offers low cost, high speed, and general availability. Furthermore, it offers the possibility to follow patients after endovascular interventions. This allows for timely recognition of restenosis before it becomes clinically apparent, and thus provides a window for secondary intervention to maintain patency. Despite these advantages in chronic settings, duplex ultrasound is not appropriate in emergent AMI for a variety of reasons. It is highly user dependent, and 24-hour availability is rare. Patients are frequently unable to cooperate because of abdominal pain, and they cannot undergo adequate fasting to minimize bowel gas. In addition, duplex images include only the first few centimeters of the SMA, and may miss a more distal embolus.
IMAGING FINDINGS
 Plain Film
Plain Film
Plain radiographs have only limited value in diagnosing patients with MI. Often findings are nonspecific and can be seen in other disease processes. In the early course plain films can show ileus with bowel distension, while late radiographs demonstrate portal venous gas, pneumatosis, or thumbprinting. Thumbprinting describes changes of the colon associated with ischemia. It is caused by thickened edematous mucosal folds that have the appearance of thumbs protruding into the intestinal lumen (Table 13–2 and Figures 13–1 through 13–3).