Fig. 9.1
The distinctions in long-term memory that are relevant to stroke and the primary supporting neural structures
Within long-term memory, there are further divisions (see previous Definitions of Memory Types and Fig. 9.1 for how the distinctions in memory are made). Memories that can be consciously recalled, such as memories for specific events (“I remember my 18th birthday party”) and facts (“The CN tower is located in Toronto”) are called declarative or explicit memories. Within explicit memory, the distinction is made between episodic and semantic memory. Episodic memory refers to the conscious recollection of an experienced event and its associated contextual details, like the time and place that the event occurred. The retrieval of an episodic memory often entails a feeling of going back and reexperiencing the event that is being recalled.
In the clinic, episodic memory is measured with tasks that require one to learn a list of stimuli (e.g., words, objects) or more complex materials (e.g., short stories or figures) and, after a delay, recall or recognize as much information as possible (see Table 9.1 for examples of common neuropsychological tests used to examine episodic memory and other types of memory). These methods are useful for studying anterograde memory, the ability to form new episodic memories. Retrograde memory, the ability to remember previous events, can be reliably assessed with tests like the Autobiographical Memory Interview [8], which evaluate the number and type of details in older episodic memories.
Table 9.1
Some common examples of neuropsychological measures used to assess memory function
Test | Age cut-off | Administration time (min) | Test description | Processes measured |
|---|---|---|---|---|
Implicit memory measures | ||||
Mirror-tracing task | – | – | Participant is asked to trace a pattern through the reflection in a mirror. Examine practice effects | Procedural memory via the improvement of mirror-tracing over time |
Serial reaction task | – | – | A dot is shown on a computer screen and the participant indicates the position. Sometimes it is random, sometimes it is predictable | Implicit learning via the time advantage between the blocks that are predictable and those that are random |
Semantic memory measures | ||||
Boston naming test (BNT) | 89 | 5 | 60 line drawings of objects and the participant is asked to name each drawing. If unable to do so, semantic and phonemic cues are provided | Integrity of the semantic network; vocabulary |
Semantic fluency tasks | 95 | 5 | Participant is asked to generate words other than proper names that belong to categories such as animals | Integrity of the semantic network; vocabulary |
Peabody picture vocabulary test | 90 | 15 | Out of four pictures, participant points to one that is described by a given word | Vocabulary |
Episodic memory measures | ||||
California Verbal Learning Tests (CVLT) | 89 | 20 | Learning, recall and recognition of a word list of related items over multiple trials | Verbal learning and retrieval strategy; acquisition rate; immediate and delay recall; interference |
Hopkins Verbal Learning Test (HVLT) | 80 | 15 | Learning, recall and recognition of a word list of related items over multiple trials | Verbal learning and retrieval strategy; acquisition rate; immediate and delay recall; interference |
Brief visual memory test (BVMT) | 70 | 15 | Recall of six designs and locations over multiple trials, recognition trial included at delay | Visual-spatial learning, recall and recognition |
Rey-Osterrieth complex figure | 93 | 15 | Construction, recall and recognition of a complex figure | Visual-spatial construction skills, learning, recall and recognition |
Doors and people test | 80 | 40 | Recall and recognition of verbal and visual information and associations | Visual and verbal memory and associative memory processes |
Autobiographical memory interview | 80 | 30 | Recall of remote personal and factual events | Episodic and semantic components of autobiographical memory |
Weschler memory scale (WMS)-III: Logical memory subtest | 89 | 15 | Immediate and Delayed recall of two short passages and delayed recognition of story details | Verbatim and gist recall and recognition of verbal narratives |
Weschler memory scale (WMS)-III: Paired associates subtest | 89 | 20 | Learning and delayed recall and recognition of a list of unrelated word pairs | Associative verbal learning, recall and recognition |
Weschler memory scale (WMS)-III: Design memory subtest | 89 | 15 | Recall item information and location of eight designs on a grid for immediate and delayed periods | Visual-spatial immediate and delayed recall of item and location information |
Whereas episodic memory entails the conscious recall of contextual events, semantic memory refers to general, context-independent knowledge. This includes memory for facts about the world (e.g., Paris is the capital of France) as well as general knowledge about ourselves (personal preference for spaghetti with tomato sauce versus alfredo sauce). Neuropsychological measures of semantic memory assess the ability to recover these factual details in formal tests (Table 9.1). For example, the category fluency task requires the generation of many items that belong to specific categories (e.g., animals) in a limited time period. The Boston Naming Test requires one to name a series of pictured objects, beginning with common objects (e.g., canoe) and ending with less common objects (e.g., abacus). In general, semantic impairment following a stroke will present as deficits in comprehension or retrieval of factual information, such as the name of an object, the president of the United States, etc. Such impairment is typically seen following damage to the left prefrontal cortex or the left posterior temporal lobe and inferior parietal cortex [9–11] (Table 9.1).
Implicit Memory
Implicit memory is a form of memory that involves the expression of knowledge without conscious awareness of how that knowledge was acquired. Remembering acquired skills, habits, and rules, such as cooking techniques, riding a bicycle, or playing chess are examples of implicit or procedural memories. Strokes in regions such as the basal ganglia and the cerebellum can result in deficits in remembering established procedural memories and in forming new ones [12]. For example, in the commonly used mirror-tracing task, an individual is asked to trace shapes using only visual feedback from a mirror. This task may be initially hard, but it becomes easier with practice. Stroke survivors with deficits in implicit memory do not benefit from practice when mirror tracing [13–15] (see Table 9.1 for other examples of measures of implicit memory). While all forms of memory are important, episodic memory processes and structures are the most likely to be affected by stroke [16]. Accordingly, the remainder of this chapter will focus on the effect of stroke on episodic memory.
Episodic Memory
Strokes causing damage to the neural network that supports episodic memory can impair the learning, storage, and retrieval of episodic memories. The nature of this impairment is dependent on where in the network damage occurs (Table 9.2).
Table 9.2
A brief summary of predicted memory impairments resulting from neuronal damage to regions of the episodic memory network
Neural damage | Vascular supply | Side | Process affected |
|---|---|---|---|
Medial temporal: Hippocampus | Posterior communicating artery; anterior choroidal artery | Left | Verbal memory; recall impaired more than recognition |
Right | Nonverbal memory; recall impaired more than recognition | ||
Medial temporal: Hippocampus and Parahippocampal regions | Posterior communicating artery; anterior choroidal artery | Left | Verbal memory; recall impairment = recognition impairment |
Right | Nonverbal memory; recall impairment = recognition impairment | ||
Thalamus: Extended hippocampal system | Polar (tuberothalamic/premammillary) artery; anterior choroidal artery | Left | Verbal memory; recall impaired more than recognition |
Right | Nonverbal memory; recall impaired more than recognition | ||
Thalamus: Medio-dorsal-perirhinal circuit | Paramedian (thalamo-perforating) artery | Left | Recall impairment = recognition impairment; executive deficits |
Right | Recall impairment = recognition impairment; executive deficits | ||
Frontal: Oribtofrontal cortex/basal forebrain | Perforating branches from anterior communicating artery, orbitofrontal artery branches of anterior (medial) and middle (lateral) cerebral arteries | Bilateral | Impaired source recall; context confusion; confabulation |
Frontal: Prefrontal cortex | Anterior cerebral artery | Left | Memory encoding and general impairment in strategic and organizational (executive) processing |
Right | Memory retrieval and general impairment in strategic and organizational (executive) processing | ||
Posterior association areas | Posterior cerebral artery, middle cerebral artery | Bilateral | Impairment in encoding and retrieving particular sensory/perceptual details |
Using a number of different methodologies, neuropsychological investigations have determined that the core of the episodic memory neural network is the hippocampus [12, 17, 18]. Subcortical and cortical structures, including the thalamus, the prefrontal cortex and posterior cortical regions, maintain dense connections with the hippocampus and other medial temporal lobe (MTL) structures. These regions mediate specific processes, which, if affected by vascular damage, bias the presentation of memory impairment in specific ways.
The Hippocampus and Episodic Memory
Appreciation of the central importance of the hippocampus (hippocampus proper, dentate gyrus, and subiculum) for episodic memory dates back to the pioneering work of Milner and her colleagues with the classic patient HM. HM suffered from intractable epilepsy and underwent bilateral MTL surgery to remove the hippocampus, parahippocampus, and related regions. The surgery relieved his epilepsy but left him with profound anterograde and retrograde amnesia for episodic events. Significantly, HM’s memory for semantic information and his implicit memory were intact [19, 20]. Since HM, considerable work has confirmed the importance of the hippocampus in episodic memory [21–25].
From these behavioral findings, theoretical frameworks, such as the Multiple Trace Theory, have implicated the hippocampus in both the encoding and retrieval stages of episodic memory [17, 26]. At encoding, the hippocampus serves the critical role of binding all the relevant memory-related details into a coherent structure across multiple brain regions. Over time, episodic memories become represented in a distributed network of subcortical and cortical brain regions. During retrieval, hippocampal neurons act as a pointer to these storage regions, initiating the process of bringing the memory into awareness.
The Hippocampus: Recollection Versus Familiarity
As indicated previously, the hippocampus is believed to be crucial for conscious recollection, a process that entails the re-experiencing and recall of an event and its associated details. Clinically, recollection is crucial for tests such as free recall and those that require the retrieval of associative information, such as where and when an event was experienced. The hippocampus is not required for memory that is based solely on familiarity. In contrast to recollection, familiarity–based memory is less detailed and usually involves remembering that an object or item has been encountered before, but without necessarily being able to recall anything else about it. Recognition tasks that require decisions based on matching memory to present information can be successfully completed with familiarity-based assessments.
Recollection impairments are present after stroke in the MTL due to, for example, hemorrhage or occlusion of the vascular supply to the hippocampus (involving the anterior and temporal artery, and to a lesser degree the anterior choroidal artery). Most strokes involving the hippocampus and MTL are ischemic; hemorrhages isolated to the hippocampus are uncommon and those in the temporal lobe are usually more lateral. Typical causes would be amyloid angiopathy or rupture of a middle cerebral artery aneurysm into the adjacent temporal lobe. Ott and Saver [27] reported cases of hippocampal lesions from strokes that were sufficient to induce amnesia (see Fig. 9.2 for a representative computed tomography [CT] scan of such a case).
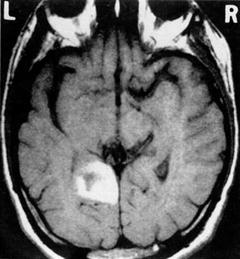

Fig. 9.2
A representative scan of one of the patients that presented with left hippocampal and parahippocampal gyrus damage. Reprinted with permission from Ott BR, Saver JL. Unilateral amnesic stroke. Six new cases and a review of the literature. Stroke. 1993 Jul;24(7):1033–42
Other investigations [24] have examined memory performance of patients who suffered from mild hypoxic ischemic encephalopathy, which is a form of transient global brain injury. Although diffuse brain regions can be affected, hypoxic ischemic encephalopathy can also selectively damage the hippocampus. Compared to healthy control participants, these patients showed memory impairment on recall and recognition memory tests; however, their recall deficit was significantly greater than their recognition deficit. Experimental measures designed to dissociate recollection from familiarity identified a selective recollection deficit in these patients [28, 29].
In 2004, Mayes and colleagues [30] described patient YR, who suffered an ischemic stroke that selectively affected both hippocampi. Consistent with the previously mentioned findings [28, 29], YR’s primary deficit reflected a loss of hippocampus-dependent memory. Her short-term, implicit and semantic memories, as well as familiarity-based recognition memory, were all spared. Her performance on tests that required recollection, such as free recall and paired-associate learning, however, was impaired.
Many of the studies that have reported anterograde amnesia after hippocampal damage also demonstrated deficits in the patients’ ability to recall details from past personal experiences (e.g., describe the first day of a job) [31]. These findings suggest that memory loss following hippocampal strokes extend to events of the distant past (retrograde amnesia). Further evidence of retrograde amnesia comes from a study that tested 20 patients with unilateral strokes, including patients who sustained damage to the hippocampus [32]. The patients were given tests of autobiographical memory in which they were asked to describe personal events from three remote time periods. They were also given tests of public events in which they answered questions about well-known events from different decades (e.g., “Who was the first person to set foot on the moon?”). The patients with hippocampal damage generated fewer details and had poorer recall of past personal events, particularly those from remote times. They also answered fewer correct responses to questions about well-known public events compared to their matched counterparts.
While recollection-specific deficits are a cardinal feature of hippocampal damage due to stroke, for example, familiarity-based memory impairment can supplement these deficits if the lesions are accompanied by damage to other MTL structures, namely the entorhinal, perirhinal, and the parahippocampal cortices. In their review, Aggleton and Shaw [33] reported, that, while focal hippocampal lesions selectively disrupt free recall (recollection), damage that extends beyond the hippocampus into other MTL regions results in recall and recognition deficits (also see [18]; but see [34] for a different view of recollection and familiarity).
The Hippocampus: Left Versus Right
Conventional laterality-specific functions are represented in the hippocampus. The left hippocampus is involved predominately in verbal episodic memory tasks whereas the right hippocampus is involved more in nonverbal episodic memory tasks, including the encoding and retrieval of spatial relations and location information. For example, poor verbal memory, as measured by story recall and tests that assess memory for a previously learned list of unrelated words, is associated with left hemisphere strokes [35]. Patients with ischemic right posterior hippocampal lesions present with relatively intact verbal memory, but have a specific impairment in nonverbal and spatial memory [36]. Szabo and colleagues [29] studied more than 50 patients using a neuropsychological battery that included a list-learning task, an auditory learning task, and tests of nonverbal memory, such as the Rey-Osterreith complex figure test. Strokes affecting the left hippocampus resulted in worse performance on the verbal memory tests (e.g., list-learning test) when tested at both short and long delays, and better performance on nonverbal memory tests (e.g., the Rey-Osterreith complex figure test). In contrast, strokes affecting right hippocampal regions resulted in the opposite pattern: poorer nonverbal than verbal memory.
The Hippocampus: Summary
The hippocampus is integral for the formation and recall of episodic memories, irrespective of when they are acquired. When the hippocampus is damaged as part of stroke-induced pathology, both anterograde and retrograde amnesia are experienced, as they are in other disease processes. If hippocampal lesions from stroke are accompanied by damage to additional regions of the MTL, familiarity-based recognition memory is also likely to be affected. Finally, the pattern of impairment from hippocampal strokes is dependent on the side of damage. Left-sided damage results in verbal memory impairments and right-sided damage results in nonverbal impairments.
The Thalamus and Episodic Memory
The role of the thalamus in episodic memory is related to its multiple connections with the MTL (Fig. 9.3). Two main circuits connect the anterior and medial dorsal thalamic nuclei to the hippocampus and parahippocampus, respectively. The anterior (and latero-dorsal) thalamic nuclei are densely connected to the hippocampus via the mammothalamic tract and the mammillary bodies forming the extended hippocampal system [33, 37]. These structures are supplied by the tuberothalamic artery, a branch of the posterior communicating artery (which also supplies anterior regions of the thalamus, including the mammillary bodies and part of the mammillothalamic tract) and the anterior choroidal arteries (the anterior nuclei complex). The medial dorsal thalamic nucleus is linked to the amygdala and the perirhinal cortex in the parahippocampal gyrus via connections that pass through the internal medullary lamina [38, 39] and also maintains reciprocal connections with the prefrontal cortices.
The thalamus is supplied by perforating arteries arising from the posterior communicating artery (polar, tuberothalamic, anterior internal optic, or premamillary artery), paramedian thalamic-subthalamic arteries from first segment of the posterior cerebral artery, inferolateral (thalamogeniculate) arteries from the second segment of the posterior cerebral artery and posterior (medial and lateral) choroidal arteries usually also from the second segment of the posterior cerebral artery. These are all derived from the vertebrobasilar arterial system, with the exception of the polar artery. Vascular supply to the medial dorsal thalamic nucleus also comes from the polar and paramedian arteries [40]. The thalamus is frequently damaged by stroke, including ischemic stroke with occlusion of the perforating arteries supplying it due to hypertension. Intracerebral hemorrhage secondary to hypertension also is common in the thalamus (Fig. 9.3).
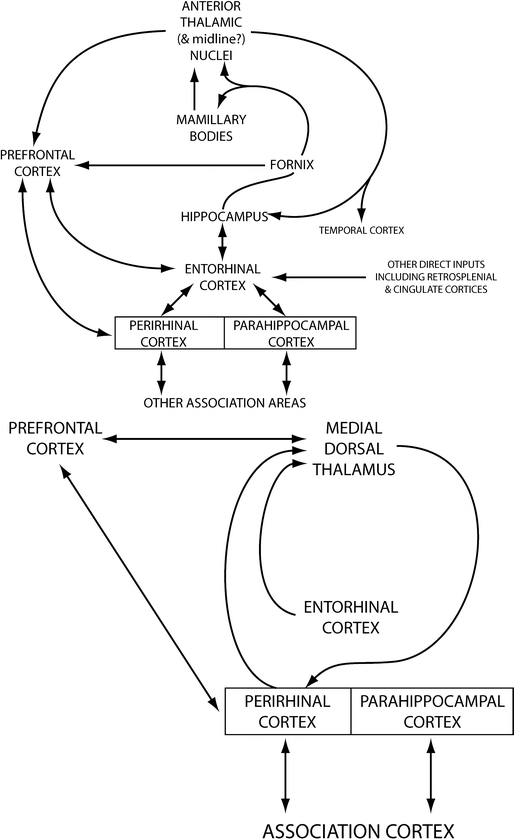

Fig. 9.3
Pathways connecting the medial temporal lobe structures to the thalamus. The top schematic represents the connections of the anterior thalamic nuclei. The bottom schematic represents the connections of the medio-dorsal nucleus of the thalamus (adapted from [33])
A pattern of memory loss that is reminiscent of hippocampal amnesia occurs following damage to the extended hippocampal system, particularly if the damage disconnects the thalamus from the hippocampus [39]. In one well-documented case, isolated injury to the left anterior thalamus following a polar thalamic infarct resulted in anterograde amnesia for episodic events, while short-term, semantic, and procedural memory as well as general intelligence remained intact [41]. As with hippocampal amnesia, in cases of thalamic stroke, there is a prominent dissociation on tests of episodic memory that is dependent on recollection and familiarity. For example, patients with thalamic stroke that damages the anterior thalamic nuclei and/or thalamic connections to the hippocampal system often present with deficits in verbal recall (recollection) memory but spared verbal recognition (familiarity) memory as measured, for example, with the California Verbal Learning Test (CVLT) [42] (also see [43–45]). Carlesimo and colleagues [46] tested a thalamic stroke patient with damage that extended to the hippocampal system. Consistent with other reports, this patient was impaired only on the recollective aspects of episodic memory on tests of anterograde and retrograde memory.
These deficits following thalamic stroke may be indistinguishable from those with hippocampal damage, yet the underlying causes of the impairment is different. Hippocampal damage results in impairment in the binding or associative processes of episodic memory at encoding and retrieval. Thalamic damage that disturbs thalamic-hippocampal pathways appears to cause impairment primarily at the encoding stage [45] in that lesions to the anterior nuclei or their afferent tracts will impair encoding new stimuli. Under ordinary conditions, the thalamus acts as a relay between the hippocampus and cortical regions of the brain where long-term memories are stored. Without these contributions, the details of the encoded memories cannot be appropriately stored, resulting in post-encoding recollection deficits [47].
Thalamic strokes can also result in executive deficits (e.g., attentional control, suppression of irrelevant information) similar to those associated with frontal-lobe damage (see Chap. 8). These deficits are usually a result of damage to the medial dorsal nucleus-perirhinal pathway from paramedian occlusions [48]. In the normal, intact brain, the reciprocal connections between the medial dorsal nucleus and the prefrontal cortex help integrate executive processes with other nonexecutive processes supported by connected brain regions. Damage to this circuit produces widespread impairment that can affect memory and a range of cognitive processes [49]. In terms of memory, patients with this pattern of damage have problems consciously remembering episodic information under conditions of high interference, often exhibiting intrusion errors and false recognitions during various types of testing [50].
The medial dorsal nucleus-perirhinal circuit appears also to be involved in familiarity-based episodic memory decisions. This is based on its connection to perirhinal cortex [33]. Supporting this notion are findings from Carlesimo and colleagues [46] who report that patients with damage to the medial dorsal nucleus-perirhinal circuit present with selective impaired familiarity, but have spared recollection [45] (see [51] for inconsistent results).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







