FIGURE 1 The fundamental principles of psychophysical assessment applied within the area of QST. A given stimulus is increased in intensity until a pain threshold (PT) or a pain tolerance threshold (PTT) is reached. Alternatively a well-defined stimulus intensity is delivered, and the pain intensity is rated on a visual analogue scale (discrete or continuous). If the thresholds or pain ratings are obtained from a pain patient with different degrees of sensitization, the thresholds are lower and the ratings higher.
One area, which has recently been developed, is selective stimulation of bone structures [6,39] and assessing periosteal sensitization [7]. This is important for obtaining better information about periosteal pain mechanism in humans as this is clinically a challenging area given that many cancer patients experience significant pain in relation to bone metastases.
Intradiscal pressure provocation [107] and external facet joint pressure stimulation [110] have been explored as potential models related to spine pain. Direct intraarticular activation of the knee joint nociceptors has been done by pressure stimulation which activates joint receptors during an arthroscopic procedure [33] which cannot be achieved by pressure applied externally to the joint [17]. Pressure algometry assesses a relatively small volume of tissue. In contrast, a larger volume can be assessed by the computer-controlled cuff-algometry technique: The pain intensity related to inflation of a tourniquet applied around an extremity is used to establish stimulus-response curves, and by this the deep-tissue pain sensitivity can be assessed [97]. The cuff is wrapped around the leg or arm and inflated in a standardized way, and the volunteer/patient stops the inflation when the pain threshold is reached or rates the pain intensity to a given standardized stimulation. The pressure applied is distributed throughout the underlying tissues activating a variety of deep somatic receptors and nociceptors (Fig. 2).
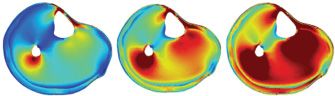
FIGURE 2 An example of a finite element estimation of the Von Mises Stress (a combination of the stress in all 3 dimensions) when the calf is stimulated with a cuff where the applied pressure is increased from low (left) to high (right) intensity. The combined stress intensity is highest around a bony structure.
It has become more and more evident that a localized nociceptive focus can drive central processes and cause contralateral or even extrasegmental wide-spread sensitization. There is ample experimental and clinical evidence that in neuropathic conditions the signs and symptoms extend into regions beyond those directly innervated by the injured nerve [63,72]. After chronic constriction injury of the sciatic nerve in rats, tactile allodynia in the hind paw territories of both the injured sciatic nerve and the uninjured saphenous nerve has been shown [120].
A simple procedure like a two-week cast immobilization causes wide-spread hyperalgesia [83]. This resembles other models where localized inflammation of one craniofacial muscle evoked mechanical allodynia in the hind paws [1] or inflammation in one hind-limb caused contralateral spreading sensitization [112]. Conditions such as migraine and chronic tension type headache also lead to spreading sensitization [36,37], and animal models have confirmed that chemical stimulation of the cranial dura elicited long-lasting hyperexcitability to innocuous (brush, pressure) and noxious (pinch, heat) stimulation of the paws [27]. Somatic pain can not only cause widespread sensitization, but also visceral hyperalgesia [74] and vice versa [43].
This widespread sensitization in otherwise localized pain problems offers a challenge in QST as a separation between the two manifestations (peripheral and central) can be difficult. An example could be pressure pain assessment over the knee joint in patients with osteoarthritis (OA) where localized joint sensitization is detected. In the same patient, the pressure pain thresholds are also reduced as compared with control subjects when assessed from the arm [17]. Similar widespread sensory manifestations of sensitization are found in e.g., unilateral epicondylitis [34,35].
This raises the issue that a true control site is most often non-existing in patients with deep chronic pain as also consistently shown in neuropathic pain [63].
The only way to overcome this lack of a control site in pain patients is to use normative databases for all the tests applied [77] and to use statistical techniques such as Z-scores to judge when an individual patient is outside the normative range [90,91].
Another aspect of spreading sensitization is that the QST platform should include sets of normally non-nociceptive stimuli as the sensitization processes may cause allodynia to e.g. thermal or mechanical stimuli and as such cold/warmth, brush, or slight pressure.
Expansion of Receptive Fields
In many animal studies, expansion of receptive fields of dorsal horn neurons has been documented in neuropathic as well as inflammatory (cutaneous, muscle, and viscera) models. Expansion of receptive fields has been a challenging mechanism to assess in humans. A nerve ligation [22] or a paw inflammation [56] led to a significant increase in the size of convergent, cutaneous receptive fields of dorsal horn neurons. Similar findings are reported after experimental myositis [54], joint inflammation [105], or sensitization of the colon [75].
In animals, an alternative method for assessment of receptive field expansion of dorsal horn neurons has been developed. This involves quantification of the so-called reflex receptive field [108,109] which was found to expand in the presence of sensitization [52,60]. This method based on assessment of the nociceptive withdrawal method has been translated to humans [3], and the reflex receptive field is found to be expanded into spinal cord injured patients [4], in patients with chronic visceral pain [76] and in patients with musculoskeletal pain [24]. Such an electrophysiological method requires advanced laboratories as compared with simple psychophysical tests, but may provide valuable information in clinical studies or in the profiling of new compounds.
Wind-Up, Temporal Summation and After-Discharge
An important and potent mechanism in dorsal horn neurons is the temporal summation mechanisms termed wind-up. Repeated strong C-fiber stimulation of nociceptive fibers causes a frequency-dependent increase in the neuronal excitability, which outlasts the stimuli [73]. The resulting response of spinal cord neurons to successive stimuli of this type is a progressive increase in the magnitude of the nociceptive input and is often followed by persistent after-discharge. Wind-up has been used as a model of neural plasticity and central sensitization in the spinal cord. It has been shown to be sensitive to NMDA receptor antagonists and implicated in a number of nociceptive responses [88]. This wind-up process as assessed by neuronal recordings from dorsal horn neurons or as nociceptive withdrawal reflex is strongly facilitated in conditions of sensitization [125].
In humans, the initial phase of the wind-up process translates into temporal summation [10]. If a painful stimulus is repeated 1-3 times per second, the pain will integrate and become more painful with an intensity and stimulus frequency dependent summation [10] (Fig. 3). Temporal summation can be elicited using electrical, mechanical, or thermal stimulation modalities and is elicited from the skin, musculoskeletal structures, and viscera [8,19].
In many clinical conditions such as neuropathic, musculoskeletal, and visceral pain, patients show a significant facilitation of temporal summation [12,49,81].
In clinical bed site testing, simple devices are used for assessing temporal summation such as tapping the skin with a nylon filament [81]. When more standardization is required, however, automated user-independent methods are needed such as thermal [62], mechanical [78], or electrical stimulation techniques of the skin [10], muscles [15], or viscera [32]. Recently, a new user-independent technique has been developed based on a tourniquet cuff (Fig. 2 and 3), which is automatically and repeatedly inflated, and the volunteer/patient rates the provoked pain intensity [68,111].
There is evidence for gender differences in temporal summation [103] that must be taken into consideration when comparing groups.
For heat stimulation there is a difference in the temporal summation assessment protocol. For heat pulses with relatively slow rise times summation is predominantly seen for second pain (C-fiber mediated) [5,79,100], whereas heat stimuli with rapid rise times, e.g. laser stimulation, summation of first pain (Aδ-fibers) can also be assessed [9].
When repeated stimuli are delivered to assess temporal summation, sometimes pain patients experience an after-sensation (pain after the stimulus has stopped) [89]. This has been observed in patient with neuropathic [47] and musculoskeletal pain [114,115]. An exclusive facilitation of the after-sensation alone has been proposed to be of diagnostic value [104] and supports the basic finding that the summation and the after-sensation are mediated by different underlying mechanisms [99,123]. There seem to be some gender differences in the experience of the after-sensation phenomenon [102].
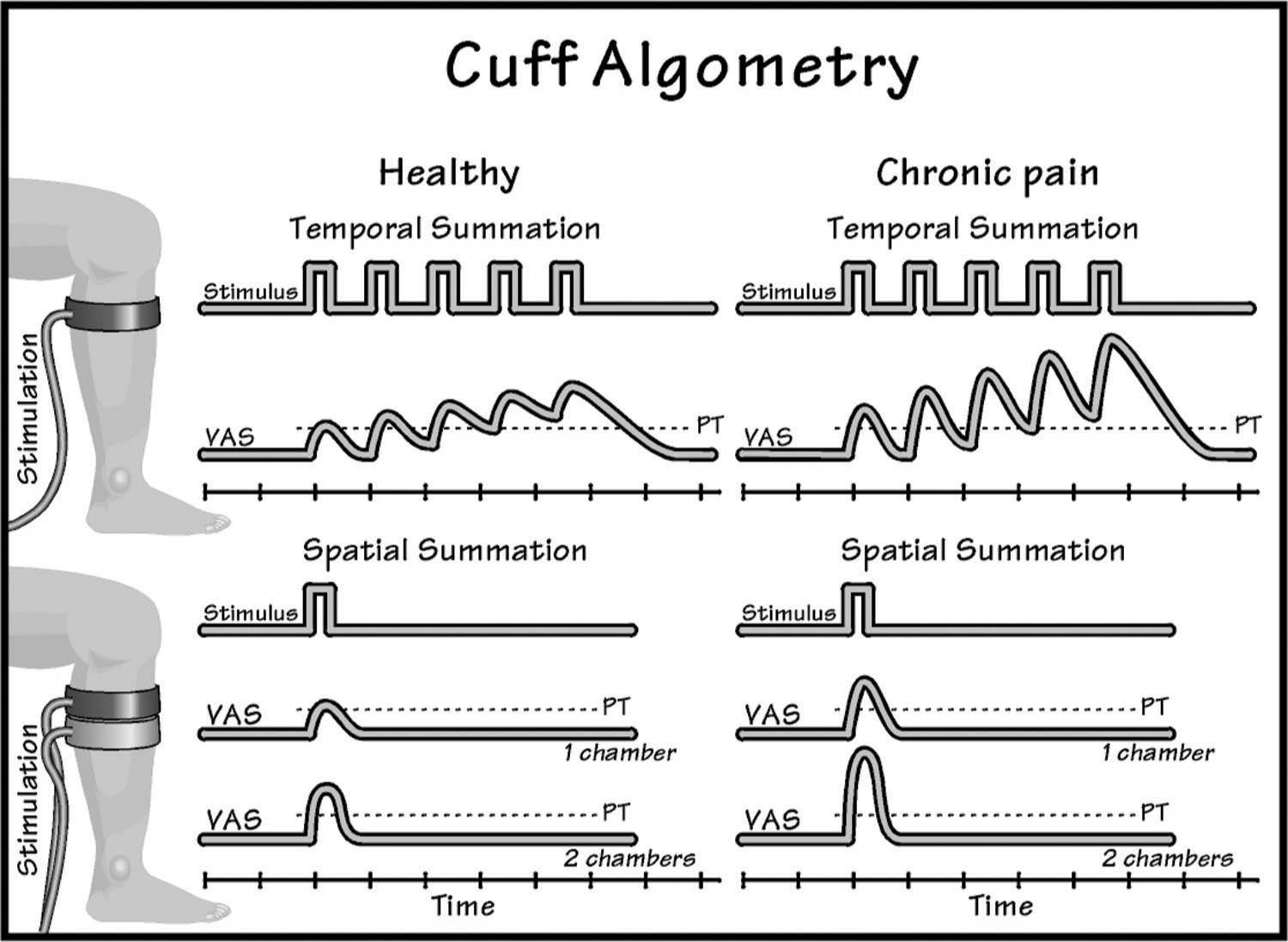
FIGURE 3 An example of how cuff algometry (inflating a cuff) can be used to assess temporal (stimuli delivered repeatedly over time) or spatial (stimuli delivered simultaneously to different areas) summation. If the time between individual stimuli is sufficiently short, temporal summation will occur. If two cuffs are activated simultaneously, stronger pain intensity will be provoked compared with single cuff stimulation.
In pain patients, temporal and spatial summation can be facilitated as a result of sensitization.
Spatial Integration
Spatial integration is another mechanism which relies on central networks and the general sensitization status [25]. In humans, spatial summation can be assessed in different ways where the stimulus is applied to using different stimulation areas e.g. using thermodes [80], pressure probes [78], or cuffs [97]. The cuff technology is the most recent where one or two cuffs can be automatically inflated in a standardized way, and the volunteer/patient rates the provoked pain intensity [97] (Fig. 3).
Spatial summation is facilitated in various pain conditions such as fibromyalgia [115,116], osteoarthritis [51], and lateral epicondylitis [59].
Descending Modulation of Neuronal Excitability
One manifestation of inhibitory influences is that associated with diffuse noxious inhibitory control (DNIC), expressed as an inhibition of dorsal horn neurons produced by a noxious stimulus applied to any body region remote from the receptive field of the neurons [76], a phenomenon recently re-termed as conditioning pain modulation [122].
In the past, the main focus has been on descending inhibition. Emerging evidence indicates, however, that descending facilitation contributes to the maintenance of neuropathic [126], inflammatory [2] and post-operative pain [127]. The descending facilitatory influences are manifested via pathways that originate in the midbrain and brainstem structures (e.g. PAG, raphe, RVM). Descending controls allow a “top-down” influence on spinal processing and form a link between higher functions such as cognition, memory, and emotions and the level of pain transmission.
There is increasing evidence that the balance between the descending inhibition and facilitation may be disturbed in chronic pain, and that this phenomenon has a role in maintaining hypersensitivity [124] alongside mechanisms of central sensitization [98, 119].
In humans, status assessment of the descending pathways has recently undergone a revival, and the original DNIC terminology has been renamed to conditioning pain modulation (CPM [122]). The “pain-inhibits-pain” paradigm (the heterotopic conditioning tonic stimuli – thermal, mechanical, electrical, or chemical) is manifest as a decrease in the pain perception evoked by a painful test stimulus presented elsewhere in the body.
Less efficient descending pain control has been reported in musculoskeletal pain conditions such as patients with e.g. myofascial temporomandibular joint pain [26], chronic low back pain [87], fibromyalgia [64], painful OA [17] and chronic tension-type headaches [101], and chronic pancreatitis [84]. OA patients with a deficient, descending pain inhibition show a normalization to a pain-free state after surgery [51,65] suggesting that the chronic pain maintained the CPM dysfunction and that the chronic pain saturated the CPM mechanism so the conditioning pain stimulus is less efficient. The mechanism of CPM seems intact in short and long-term rheumatoid arthritis patients compared with control subjects [67]. A dysfunction of descending pain modulation mechanisms in e.g. fibromyalgia (reduced activation of the rostral anterior cingulate cortex [58]) may contribute to the clinical manifestations of widespread pain. Evidently, an alteration in the descending pain modulation could be a promising target for pharmacological intervention [19].
An impaired CPM response can be interpreted as reduced inhibition but alternatively it could also be the result of increased facilitation. At this stage no diagnostic technique has been developed to separate those two competing pathways. Since impaired CPM may appear as spreading hypersensitivity which may also link to sensitization of central mechanisms, it is important to combine assessment of different mechanisms, e.g. temporal summation of pain along with assessment of CPM. Unfortunately, the CPM paradigms currently used show high variability [86], and hence the technology needs further development and refinement before it can be applied for diagnostics or drug profiling [13].
Provocation of Referred Pain
True referred pain is a phenomenon predominantly related to pain from deep somatic and visceral structures. Referred pain can occur as a result of a given pathology (e.g. ureteral calculosis, dysmenorrhea) or by provocation of a given structure (e.g. active myofascial trigger points). Referred pain is the result of a central mechanism, and when experimentally evoked, it can be facilitated in patients with e.g. fibromyalgia [113], whiplash [61], osteoarthritis [20], chronic low back pain [85
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







