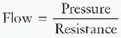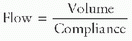The lung-ventilator unit can be thought of as a tube with a balloon network on the end, with the tube representing the ventilator tubing, endotracheal tube (ETT), and airways and the balloon network representing the alveoli.
The lung-ventilator unit can be thought of as a tube with a balloon network on the end, with the tube representing the ventilator tubing, endotracheal tube (ETT), and airways and the balloon network representing the alveoli. To generate gas flow, the total forces must overcome the resistive forces of the airway, the ventilator tubing, and the ETT to allow gas to flow into or out of the lung, depending on driving pressure gradients.
To generate gas flow, the total forces must overcome the resistive forces of the airway, the ventilator tubing, and the ETT to allow gas to flow into or out of the lung, depending on driving pressure gradients. The equation of motion for the respiratory system relates how volume (ΔV), pressure (ΔP), and flow ([V with dot above])interact to move gas into and out of the lung:
The equation of motion for the respiratory system relates how volume (ΔV), pressure (ΔP), and flow ([V with dot above])interact to move gas into and out of the lung: The manner in which each variable (pressure, volume, or flow) is controlled, described as the mode of ventilation, determines how the ventilator delivers the mechanical breath.
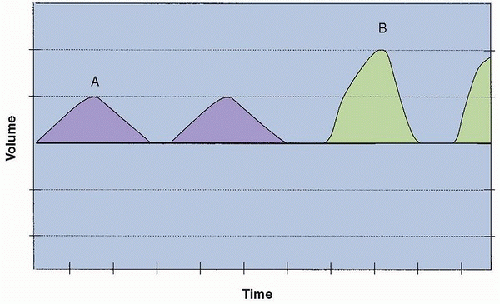
The manner in which each variable (pressure, volume, or flow) is controlled, described as the mode of ventilation, determines how the ventilator delivers the mechanical breath. In pressure-control (PC) ventilation, the pressure pattern is “square,” but flow increases rapidly at the beginning of the inspiratory phase to generate the set pressure limit and then decays exponentially over the inspiratory time. This flow pattern is described as decelerating flow.
In pressure-control (PC) ventilation, the pressure pattern is “square,” but flow increases rapidly at the beginning of the inspiratory phase to generate the set pressure limit and then decays exponentially over the inspiratory time. This flow pattern is described as decelerating flow. Clinicians often choose PC ventilation in patients with poor compliance, although the true benefit of PC versus other modes has not been well established in animal or clinical studies.
Clinicians often choose PC ventilation in patients with poor compliance, although the true benefit of PC versus other modes has not been well established in animal or clinical studies. If the clinician sets volume as a function of time, pressure then varies with compliance. Volume is the independent variable and pressure is the dependent variable.
If the clinician sets volume as a function of time, pressure then varies with compliance. Volume is the independent variable and pressure is the dependent variable. Understanding the factors involved in tidal volume (VT) measurement and how they are determined in different ventilators and techniques is important in patient management.
Understanding the factors involved in tidal volume (VT) measurement and how they are determined in different ventilators and techniques is important in patient management. The volume of gas that becomes compressed within the ventilator tubing and never reaches the patient is termed the compressible volume of the circuit.
The volume of gas that becomes compressed within the ventilator tubing and never reaches the patient is termed the compressible volume of the circuit. When compression volume is accounted for in determination of patient VT, the resultant volume is termed the effective tidal volume (eVT), which means that this is the VT that reaches the patient’s lungs.
When compression volume is accounted for in determination of patient VT, the resultant volume is termed the effective tidal volume (eVT), which means that this is the VT that reaches the patient’s lungs. While measuring VT at the airway opening may give the best estimate of VT in the lungs, this can be associated with problems such as losing air around uncuffed ETTs, unreliable measuring sensors, and potential extubation from the weight of the measuring device.
While measuring VT at the airway opening may give the best estimate of VT in the lungs, this can be associated with problems such as losing air around uncuffed ETTs, unreliable measuring sensors, and potential extubation from the weight of the measuring device. The manner in which each variable (pressure, volume, or flow) is controlled, described as the mode of ventilation, determines how the ventilator delivers the mechanical breath.
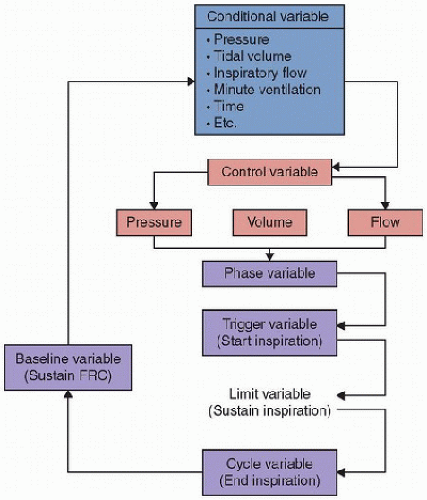
The manner in which each variable (pressure, volume, or flow) is controlled, described as the mode of ventilation, determines how the ventilator delivers the mechanical breath. Control variables are the independent variables, which arer pressure, volume, or flow. Conditional variables are determinants of a response to a preset threshold, which are both clinician set and influenced by dependent and independent variables. Phase variables are those that are used to start, sustain, and end the phase.
Control variables are the independent variables, which arer pressure, volume, or flow. Conditional variables are determinants of a response to a preset threshold, which are both clinician set and influenced by dependent and independent variables. Phase variables are those that are used to start, sustain, and end the phase. Most ventilators cannot directly measure volume; rather, they calculate volume delivered from flow that occurs over a period of time.
Most ventilators cannot directly measure volume; rather, they calculate volume delivered from flow that occurs over a period of time. Studies have shown that the ventilator-displayed VT, without software compensation for circuit compliance, generally overestimates the true delivered VT. Conversely, when the circuit-compliance compensation feature is on, the ventilator-displayed VT generally underestimates the true delivered VT.
Studies have shown that the ventilator-displayed VT, without software compensation for circuit compliance, generally overestimates the true delivered VT. Conversely, when the circuit-compliance compensation feature is on, the ventilator-displayed VT generally underestimates the true delivered VT. The neurally adjusted ventilatory assist approach to mechanical ventilation is based on the acquisition of the patient’s neural respiratory output as it is transmitted through the phrenic nerve to the diaphragm.
The neurally adjusted ventilatory assist approach to mechanical ventilation is based on the acquisition of the patient’s neural respiratory output as it is transmitted through the phrenic nerve to the diaphragm. In infants, the fact that lung volume may increase at a greater rate than airway diameter may be one reason why infants are especially prone to air trapping and hyperinflation.
In infants, the fact that lung volume may increase at a greater rate than airway diameter may be one reason why infants are especially prone to air trapping and hyperinflation. The high compliance and low elastic recoil of the infant/young child’s chest wall result in increased work of breathing to move the same VT as an adult.
The high compliance and low elastic recoil of the infant/young child’s chest wall result in increased work of breathing to move the same VT as an adult. Patient-ventilator asynchrony is a result of factors related to ventilator triggering, adequacy of flow delivery, adequate breath termination, and effects of PEEP and/or intrinsic PEEP (PEEPi).
Patient-ventilator asynchrony is a result of factors related to ventilator triggering, adequacy of flow delivery, adequate breath termination, and effects of PEEP and/or intrinsic PEEP (PEEPi). It is important to recognize that the goal of mechanical ventilatory support is not to normalize the patient’s blood gases at the cost of ventilator-induced lung injury.
It is important to recognize that the goal of mechanical ventilatory support is not to normalize the patient’s blood gases at the cost of ventilator-induced lung injury. For patients with obstructive lung disease, lung protection may include a lower VT and prolonged exhalation times. A support mode with spontaneous breathing may also be useful.
For patients with obstructive lung disease, lung protection may include a lower VT and prolonged exhalation times. A support mode with spontaneous breathing may also be useful. In patients with poor compliance, maintaining lung recruitment without the use of excessive distending volume, changes in pressure to distend the alveoli, and end-expiratory pressure may limit ventilator-induced lung injury.
In patients with poor compliance, maintaining lung recruitment without the use of excessive distending volume, changes in pressure to distend the alveoli, and end-expiratory pressure may limit ventilator-induced lung injury. Lung injury prevention is directed toward reducing cyclic collapse and re-expansion of alveoli due to inadequate PEEP.
Lung injury prevention is directed toward reducing cyclic collapse and re-expansion of alveoli due to inadequate PEEP. Widespread use of low VTs may result in reabsorption atelectasis in basal lung areas. This effect can be enhanced by the use of high-inhaled O2 concentrations. PEEP may be protective in these circumstances.
Widespread use of low VTs may result in reabsorption atelectasis in basal lung areas. This effect can be enhanced by the use of high-inhaled O2 concentrations. PEEP may be protective in these circumstances. It is important not to be misled by an improvement of oxygenation alone as a measure of lung recruitment. An increase in PEEP can reduce cardiac output and increase PaO2 despite a decrease in O2 delivery.
It is important not to be misled by an improvement of oxygenation alone as a measure of lung recruitment. An increase in PEEP can reduce cardiac output and increase PaO2 despite a decrease in O2 delivery. The effect of PEEP is as a distending pressure to increase the functional residual capacity (volume of gas at the end of exhalation in the lung).
The effect of PEEP is as a distending pressure to increase the functional residual capacity (volume of gas at the end of exhalation in the lung). The peak inspiratory pressure in PC ventilation is usually lower than in volume-control (VC) ventilation.
The peak inspiratory pressure in PC ventilation is usually lower than in volume-control (VC) ventilation. PC ventilation does not guarantee minute ventilation. Worsened compliance or resistance in the patient will result in decreased VT.
PC ventilation does not guarantee minute ventilation. Worsened compliance or resistance in the patient will result in decreased VT. The constant-flow pattern in VC may not meet patient demands, especially in patients with poorly compliant lungs.
The constant-flow pattern in VC may not meet patient demands, especially in patients with poorly compliant lungs. Airway-pressure-release ventilation (APRV) is a high-level continuous positive-airway pressure mode that is terminated for brief periods.
Airway-pressure-release ventilation (APRV) is a high-level continuous positive-airway pressure mode that is terminated for brief periods. Dexamethasone, heliox, and noninvasive ventilation may be helpful in preventing intubation or reintubation in patients with respiratory illness.
Dexamethasone, heliox, and noninvasive ventilation may be helpful in preventing intubation or reintubation in patients with respiratory illness. Bulk convection, pendelluft, Taylor dispersion, convective dispersion, cardiogenic mixing, collateral ventilation, and molecular diffusion are distinct modes of gas exchange that interact with each other during high-frequency oscillation ventilation (HFOV).
Bulk convection, pendelluft, Taylor dispersion, convective dispersion, cardiogenic mixing, collateral ventilation, and molecular diffusion are distinct modes of gas exchange that interact with each other during high-frequency oscillation ventilation (HFOV). Increasing the mean airway pressure increases alveolar recruitment and oxygenation during HFOV.
Increasing the mean airway pressure increases alveolar recruitment and oxygenation during HFOV. Alveolar ventilation during HFOV is a function of the rate of oscillations and the squared VT (VCO2 = f × VT2).
Alveolar ventilation during HFOV is a function of the rate of oscillations and the squared VT (VCO2 = f × VT2). In HFOV, VT delivery and CO2 elimination are directly related to the peak-to-trough pressure amplitude, and negatively correlated with device frequency.
In HFOV, VT delivery and CO2 elimination are directly related to the peak-to-trough pressure amplitude, and negatively correlated with device frequency. of effort provided by the patient’s muscles during spontaneous breathing attempts and support derived from the ventilator. The movement of gas is determined by forces, displacements, and the rate of change of component displacements, which are distensible. To generate a volume displacement, the total forces have to overcome both the elastic and resistive elements of the lung and airway/chest wall. To generate gas flow, the total forces must overcome the resistive forces of the airway, the ventilator tubing, and the ETT to allow gas to flow into or out of the lung, depending on driving pressure gradients.
of effort provided by the patient’s muscles during spontaneous breathing attempts and support derived from the ventilator. The movement of gas is determined by forces, displacements, and the rate of change of component displacements, which are distensible. To generate a volume displacement, the total forces have to overcome both the elastic and resistive elements of the lung and airway/chest wall. To generate gas flow, the total forces must overcome the resistive forces of the airway, the ventilator tubing, and the ETT to allow gas to flow into or out of the lung, depending on driving pressure gradients. generate these forces and how these controllers interface with the patient will then be examined. Finally, the indications, settings, and modes of ventilatory support available to clinicians who care for infants and children will be discussed.
generate these forces and how these controllers interface with the patient will then be examined. Finally, the indications, settings, and modes of ventilatory support available to clinicians who care for infants and children will be discussed.and chest wall expansion. We commonly measure the result of these forces as the airway pressure (PAWO). Opposing pressures to lung and chest wall expansion are the sum of elastic recoil pressure (Pelastic), flow resistive pressure (Presistive), and inertance
 pressure (Pinertance) within the respiratory system. Thus,
pressure (Pinertance) within the respiratory system. Thus,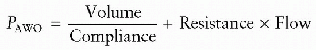
 variables, while other important factors such as resistance and compliance are dependent upon the resistive and elastic properties of the respiratory system and cannot be directly
variables, while other important factors such as resistance and compliance are dependent upon the resistive and elastic properties of the respiratory system and cannot be directly  controlled.
controlled. determines how the ventilator delivers the mechanical breath. In the equation of motion, the form of any of the variables (pressure, volume, and flow are expressed as functions of time) can be predetermined. This principle serves as the theoretical basis for classifying ventilators as pressure, volume, or flow controllers (Fig. 38.1). The necessary and sufficient criteria for determining which variable is controlled are listed in Table 38.1. It is important to recognize that any ventilator can only directly control one variable—pressure, volume, or flow—at a time. Thus, a ventilator is simply a technology that controls the airway pressure waveform, the inspired volume waveform, or the inspiratory flow waveform, and pressure, volume, and flow are referred to in this context as control variables.
determines how the ventilator delivers the mechanical breath. In the equation of motion, the form of any of the variables (pressure, volume, and flow are expressed as functions of time) can be predetermined. This principle serves as the theoretical basis for classifying ventilators as pressure, volume, or flow controllers (Fig. 38.1). The necessary and sufficient criteria for determining which variable is controlled are listed in Table 38.1. It is important to recognize that any ventilator can only directly control one variable—pressure, volume, or flow—at a time. Thus, a ventilator is simply a technology that controls the airway pressure waveform, the inspired volume waveform, or the inspiratory flow waveform, and pressure, volume, and flow are referred to in this context as control variables.TABLE 38.1 VENTILATOR CONTROLLERS | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
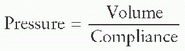

and maintained constant throughout inspiration. Pressure and VT will vary with time, depending on the compliance and resistance.
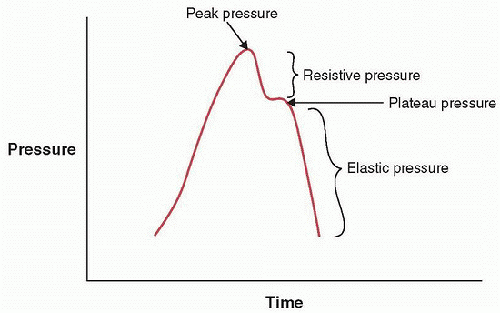 FIGURE 38.3. Pressure-time waveform from a constant-flow mode illustrating resistive and elastic elements of the respiratory system. |
to generate the set pressure limit, then decays exponentially over the inspiratory time. This flow pattern is described as decelerating flow. It is said that the major difference between volume and pressure ventilation is based on the square-wave and the decelerating flow patterns observed. In PC mode, the initial “snap” of high flow to reach the set pressure limit has been thought to be potentially beneficial in opening stiff alveoli in conditions such as acute respiratory distress syndrome (ARDS) or surfactant deficiency. It has been proposed that a decelerating flow favors better gas exchange and improves distribution of ventilation among lung units with heterogeneous time constants. For this reason, clinicians often choose PC ventilation in patients with poor compliance, although the true benefit of PC versus other modes has not been well established in animal or clinical studies.
 elastic components can be rearranged to display how volume is determined:
elastic components can be rearranged to display how volume is determined: level of precision is essential to optimize ventilator settings using lung-protective techniques. During the inflation phase of mechanical ventilation, pressure rises within the ventilator circuit, causing elongation and distension of the tubing and compression of the gas within the circuit. The volume of the gas, which becomes compressed within the ventilator tubing and never reaches the patient, is termed the compressible volume of the circuit. While in most circumstances, this volume is standard within different sizes of circuits, variation can occur. Knowing the compressible volume within the ventilator circuit is important in determining the actual VT being delivered to the patient’s lungs. When compression volume is accounted for in determining patient VT, the resultant volume is termed
level of precision is essential to optimize ventilator settings using lung-protective techniques. During the inflation phase of mechanical ventilation, pressure rises within the ventilator circuit, causing elongation and distension of the tubing and compression of the gas within the circuit. The volume of the gas, which becomes compressed within the ventilator tubing and never reaches the patient, is termed the compressible volume of the circuit. While in most circumstances, this volume is standard within different sizes of circuits, variation can occur. Knowing the compressible volume within the ventilator circuit is important in determining the actual VT being delivered to the patient’s lungs. When compression volume is accounted for in determining patient VT, the resultant volume is termed  the effective tidal volume (eVT), which means that this is the VT that reaches the patient’s lungs. eVT can be calculated thus:
the effective tidal volume (eVT), which means that this is the VT that reaches the patient’s lungs. eVT can be calculated thus: conventional ventilator is caused by several factors, including (a) difficulty of compensating for volume loss in the ventilator circuit or in the humidifier and (b) changes in temperature, humidification, and secretions, which may also influence the amount of gas that gets delivered from the ventilator to the actual patient. Air leaks around the ETT itself, especially in small patients with uncuffed tubes, are another source of volume measurement error. Measuring VT at the proximal airway eliminates most circuit compliance and other dead-space factors. Therefore, it has been recommended that the proximal airway be the only site at which to obtain accurate volume measurements in infants and children (4). To measure volumes at the proximal airway, a pneumotachograph must be positioned at the patient’s airway opening or at the ETT. Unfortunately, this technique has disadvantages that are especially apparent in infants and children. A pneumotachograph placed at the proximal ETT opening creates dead space of its own, which can be detrimental in infants who already have small VTs (5). In addition, the proximally placed pneumotachograph may impair admittance to the ETT and airways and make suctioning more difficult. Secretions can also result in contamination of the pneumotachograph and distort observed measurements. Finally, the weight of the pneumotachograph at the proximal end of the ETT increases its overall weight and may result in an increased risk of extubation.
conventional ventilator is caused by several factors, including (a) difficulty of compensating for volume loss in the ventilator circuit or in the humidifier and (b) changes in temperature, humidification, and secretions, which may also influence the amount of gas that gets delivered from the ventilator to the actual patient. Air leaks around the ETT itself, especially in small patients with uncuffed tubes, are another source of volume measurement error. Measuring VT at the proximal airway eliminates most circuit compliance and other dead-space factors. Therefore, it has been recommended that the proximal airway be the only site at which to obtain accurate volume measurements in infants and children (4). To measure volumes at the proximal airway, a pneumotachograph must be positioned at the patient’s airway opening or at the ETT. Unfortunately, this technique has disadvantages that are especially apparent in infants and children. A pneumotachograph placed at the proximal ETT opening creates dead space of its own, which can be detrimental in infants who already have small VTs (5). In addition, the proximally placed pneumotachograph may impair admittance to the ETT and airways and make suctioning more difficult. Secretions can also result in contamination of the pneumotachograph and distort observed measurements. Finally, the weight of the pneumotachograph at the proximal end of the ETT increases its overall weight and may result in an increased risk of extubation.effective VT was not statistically different from the ventilator-measured VT in PC mode; however, individual differences were large (range: -26% to +52%). The calculated effective VT was less than the VT measured at the ETT in the VC mode, with large individual differences (median: -23%, range: -48% to +21%). All of these clinical studies have recommended that the VT should be measured at the ETT in infants and small children. As stated previously, the effective VT can be calculated as the ventilator-measured expired VT. However, this method fails to take into account volume lost internally in the ventilator. Manufacturers have attempted to compensate for these volume losses by measuring compression volume loss in the system. The compliance factor can be calculated as
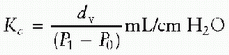
 is on, the ventilator-displayed VT generally underestimates the true delivered VT.
is on, the ventilator-displayed VT generally underestimates the true delivered VT.from triggering until the maximum flow of gas occurs. The most common trigger variables are time and flow.
 assist (NAVA) (14). The NAVA approach to mechanical ventilation is based on the acquisition of the patient’s neural respiratory output as it is transmitted through the phrenic nerve to the diaphragm. This signal is acquired via an esophageal catheter with an imbedded series of electrodes that capture the electrical activity signal of the diaphragm, known as Edi. NAVA responds by providing the requested level of ventilatory support to the patient from the Edi. The advantage of this system is the ability to acquire the patient’s desire to trigger the ventilator quickly and to offer feedback between patient effort and ventilator output. At this writing, NAVA is being investigated in clinical trials in Europe in neonatal, pediatric, and adult patients.
assist (NAVA) (14). The NAVA approach to mechanical ventilation is based on the acquisition of the patient’s neural respiratory output as it is transmitted through the phrenic nerve to the diaphragm. This signal is acquired via an esophageal catheter with an imbedded series of electrodes that capture the electrical activity signal of the diaphragm, known as Edi. NAVA responds by providing the requested level of ventilatory support to the patient from the Edi. The advantage of this system is the ability to acquire the patient’s desire to trigger the ventilator quickly and to offer feedback between patient effort and ventilator output. At this writing, NAVA is being investigated in clinical trials in Europe in neonatal, pediatric, and adult patients.
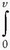 P is the integral of the pressure across the respiratory system as a function of volume, and dv is the change in the volume of the respiratory system.
P is the integral of the pressure across the respiratory system as a function of volume, and dv is the change in the volume of the respiratory system.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree