KEY POINTS
The critically ill traveler can provide a diagnostic dilemma for the clinician given the wide array of causative agents.
The patient’s travel history can lay a foundation for an epidemiological-based approach to therapy.
Certain infectious agents that respond to antimicrobial therapy must be considered early, with rapid administration of the appropriate treatment medications. These include malaria, rickettsial disease, meningococcus, plague, tularemia, and influenza.
Viral syndromes such as Middle East respiratory syndrome coronavirus (MERS-CoV), viral hemorrhagic fever (VHF), Ebola, and dengue are managed with supportive care only, as there are no available treatment medications.
The management of the critically ill traveler includes early isolation and HCW protection should be initiated until a diagnosis can be determined.
INTRODUCTION
International travel is a fact of modern life. In 2000, nearly 700 million people worldwide visited a separate country from their residence.1-3 In 2006, roughly 30 million US citizens left the country and in 2007, 14% of the US population made a total of 64 million trips outside the borders of the USA.4-8 First- and second-generation immigrants in the developed world, who return to countries of origin while visiting friends and relatives, constitute up to 40% of all travelers from the United States.9
Both returning travelers and local visitors can present with disease related to travel. Much of this disease will be present on arrival, or develop shortly thereafter. Only a minority will occur while undergoing travel, requiring a return to the home country, and of these returns, an even smaller minority will be critically ill.4,6,7 Of 100,000 travelers to the developing world, roughly 300 will undergo hospitalization, 50 will be air evacuated, and 1 will die.2,3 The major causes of mortality and serious morbidity associated with travel are cardiovascular disease and trauma sustained from motor vehicle accidents.2,3,5 Studies performed in the late 20th century suggest that infectious diseases account for less than 5% of travel-associated mortality.4,6,7 Trends in international migration and travel, however, are likely to cause an increase in people returning to the developing world with severe infections. Currently 50 million people from developed countries visit the developing world yearly, and this number appears to be increasing.2,3,10 In addition, preliminary data suggest that visitors and expatriates are expanding subpopulations with increased risk for both injurious and infectious consequences of international travel.8,9,11 More recent estimates state that 8% of travelers to the developing world seek medical attention for infectious illness.1,12-14 While the management of critical trauma and cardiovascular disease can be difficult, the varying exposures and subsequent infectious diseases associated with critical illness present the most difficult cases for the critical care practitioner.
This chapter offers an approach to the critically ill traveler, ranging from a broad empiric evaluation and treatment strategy through common disease to subsequent public health protection and impact.
THE IMPORTANCE OF THE CRITICALLY-ILL TRAVELER
The rate of illness after travel is unknown, but some self-reported rates suggest 22% to 64% of travelers to developing countries suffer some sort of illness related to travel. GeoSentinel, the global surveillance network of the International Society of Travel Medicine and the Centers for Disease Control and Prevention (CDC), publishes on travel-related illness by category and location. Sentinel data on ill travelers are collected at more than 40 GeoSentinel sites on six continents. In 2006, a clinical-based surveillance study on 17,353 ill travelers who returned from travel in developing countries was reported from 30 sites on 6 continents via GeoSentinel.4,6,7 The report covered June 1996 to August 2004. The primary manifestations for approximately two-thirds of the returned travelers fell into five major syndrome categories: systemic febrile illness without localizing findings, acute diarrhea, dermatologic disorders, chronic diarrhea, and nondiarrheal gastrointestinal disorders. A later report from GeoSentinel specifically evaluated fever, which was the reason for seeking care in 28% of almost 25,000 ill returning travelers seen between 1997 and 2006.4,6,7 The most common specific diagnoses among patients with fever were malaria and dengue fever (21% and 6% of cases, respectively). Twenty-two percent of patients had an unspecified febrile illness that was not identified while febrile diarrheal disease occurred in 15% of patients. Fever and respiratory infection were seen in 14% of patients. Almost 70% of sick travelers at GeoSentinel sites had visited sub-Saharan Africa, Southeast Asia, the Caribbean, and Central and South America. However, the rate of critical illness and death was not determined by these studies, and, in fact, may be falsely low given the low rate of self-reporting among severely ill patients.4,6,7
Management of a febrile, critically ill traveler can be difficult, especially given the wide range of infectious agents that can cause disease. Many of these entities, uncommon in the developed world, present without specific symptoms or signs and may not seem temporally related to travel itself. Rare and unusual diseases are becoming more common, and their presenting symptoms and clinical patterns may be unfamiliar to health care providers in the developed world. In addition, diagnosis often requires ancillary testing not available to all hospitals and as a consequence may be delayed or require specialty public health laboratories. Finally, decisions concerning appropriate antimicrobial therapy as well as infection control measures are optimally made early in the course of the disease, often before clinical trajectory is known and diagnostic information has returned.
However, only a few diseases and organisms need early recognition and treatment.4,6,7 Table 77-1 identifies the most common pathogens that cause illnesses in travelers, ranging from the common but self-limiting diarrhea through more rare but deadly sources of acute respiratory failure. Only a small number of these cases, however, are found within the ICU. Severe (including cerebral) malaria, meningococcal meningitis, dengue fever, viral hemorrhagic fevers (eg, Ebola, Marburg), severe coronaviruses (SARS and MERS-CoV), influenza, plague, and tularemia are lethal pathogens causing rapid multiorgan system failure in a traveler.4,6,7 Thus, despite the wide array of etiologic possibilities, a systematic approach to evaluation and empiric therapy, with definitive diagnostics, will allow for an efficient, organized care plan for the traveling patient.
DIAGNOSTIC APPROACHES AND EARLY THERAPEUTICS
The rate of critical illness among the sick traveler is largely unknown, but most likely occurs in a minority of cases. Given the wide array of clinical possibilities, and the time-sensitive nature required in critically ill patients with recent travel, epidemiologic clues provide the initial pathway of treatment. When giving consideration to the more uncommon diseases, however, it is of paramount importance that investigation and therapeutic intervention are also directed toward less exotic community acquired pathogens.1,4,6,7,13 These epidemiologic steps are the first clues to potential disease, with physical examination findings and laboratory/radiology studies providing additional support for the diagnosis or path of empiric treatment. In essence, both rare and common illnesses should be considered in a critically ill traveler.
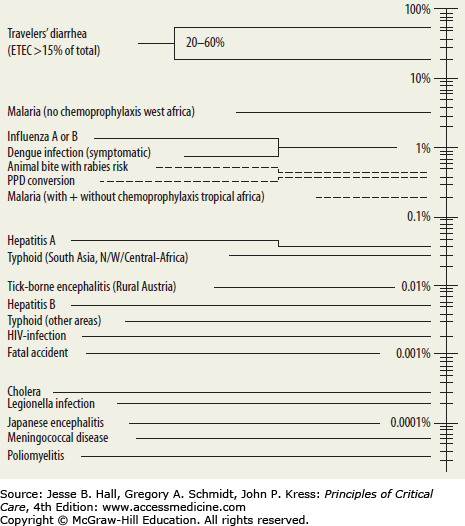
A detailed travel history is essential for the identification of the pathogen that has led to critical illness.1,4,6,7,13 Table 77-2 outlines the major diseases of travelers by region. Careful attention must be paid to all destinations, the seasons of visitation, and the extent of pretravel vaccination and prophylaxis. Expatriates and visitors are more likely to forego prophylactic measures appropriate for their sites of travel. Recently, data from 30 travel and tropical medicine sites on six continents have been integrated to enhance our understanding of common pathogens encountered by travelers.1 Based on these data, a few important generalizations can be made. First, malaria is among the top three pathogens causing severe illnesses in virtually all developing areas of the world, and is the most common infection in all sub-Saharan Africa.1,4,6,7,13 Second, dengue is an extremely common cause of illness from all developing areas outside of sub-Saharan Africa, followed by rickettsial diseases.
Diseases Causing Critical Illness in the Traveler by Geographic Distribution
| Area of Origin | Endemic Organism/Syndrome |
|---|---|
| Sub-Saharan Africa | Malaria (falciparum) |
| Acute diarrhea | |
| Dengue | |
| Influenza | |
| Schistosomiasis (mansoni, haematobium) | |
| Meningococcal meningitis | |
| Trypanosomiasis | |
| Chikungunya | |
| Viral hemorrhagic fever (Ebola/Marburg, Rift Valley fever) | |
| Histoplasmosis | |
| Anthrax | |
| Plague | |
| Endemic typhus | |
| East and Southeast Asia | Acute diarrhea |
| Dengue | |
| Malaria (drug resistant) | |
| Influenza | |
| Schistosomiasis (japonicum) | |
| Tick-born encephalitis | |
| Melioidosis | |
| Japanese encephalitis | |
| Anthrax | |
| Plague | |
| SARS | |
| Endemic typhus | |
| Central Asia | Acute diarrhea |
| Dengue | |
| Malaria | |
| Influenza | |
| South and Central America and Caribbean | Acute diarrhea |
| Dengue | |
| Malaria (falciparum) | |
| Influenza | |
| Histoplasmosis | |
| Coccidioidomycosis | |
| Yersinia, schistosomiasis | |
| Anthrax | |
| Plague | |
| Endemic typhus | |
| Worldwide distribution | Pneumococcus |
| Influenza | |
| Leptospirosis | |
| Hepatitis A/E | |
| Rabies | |
| Legionella |
The length of time between visitation to each destination and symptom onset can also provide useful insight as to etiology of severe illness. Table 77-3 outlines the average incubation period of these major illnesses. Most entities have symptom onset within 7 to 10 days of exposure, though some diseases like Plasmodia falciparum and acute schistosomiasis may have a delay in onset between 1 and 2 months.1,7,8,11,15 Pointed inquiry concerning the nature of environments encountered (urban, rural, cruise, adventure travel) as well as direct exposure history (animals, fleas, sick contacts, untreated water) is also very important in diagnosis (Table 77-4).1,7,8,11,15 Additional behaviors, from contact with wildlife and domesticated animals to sexual activity, are essential data points. However, many critically ill patients cannot provide this history due to their severity of illness (obtundation, respiratory failure with mechanical ventilation, sedation, agitation) and thus, epidemiologic investigations can be difficult in these settings.
Incubation periods of common pathogens causing critical illness
| <10 Days | 11-30 Days | >30 Days |
|---|---|---|
| Dengue | Malaria (falciparum) | Malaria (falciparum) |
| Viral hemorrhagic fever | Leptospirosis | Schistosomiasis |
| Rickettsial disease | Rickettsial disease | Paragonimiasis |
| Yersinia | Strongyloides | Hepatitis A/E |
| Influenza | Hepatitis A/E | |
| Anthrax | ||
| Hantavirus | ||
| Melioidosis | ||
| Legionella |
Pathogens Causing Critical Illness in Travelers Organized by Exposure
| Exposure/Environment | Associated Disease |
|---|---|
| Urban | Dengue |
| Leptospirosis | |
| Rural | Plague |
| Mosquitoes (R) | Viral hemorrhagic fever |
| Dengue | |
| Malaria | |
| Fleas, mites | Plague |
| Endemic typhus | |
| Animal | Rabies (street dogs, bats, cats, monkeys) |
| Plague (rodents, rabbits, animal carcasses) | |
| Anthrax (carcasses, goatskins) | |
| Herpes B virus (monkeys) | |
| Hantavirus | |
| Influenza (birds) | |
| Histoplasmosis (bats) | |
| Endemic typhus (flying squirrel) | |
| Fly, ticks | African trypanosomiasis |
| Rocky Mountain spotted fever | |
| Sand/dirt | Leptospirosis |
| Coccidioidomycosis | |
| Histoplasmosis | |
| Fresh water swimming | Leptospirosis |
| Schistosomiasis | |
| Adventure travel/eco travel/hunting | Leptospirosis |
| Histoplasmosis (spelunking) | |
| Schistosomiasis | |
| Melioidosis | |
| Rocky Mountain Spotted fever | |
| IVDA/piercing/blood products/acupuncture | HIV |
| Hepatitis A/E | |
| Sick contacts | Influenza |
| Meningococcus | |
| Viral hemorrhagic fever | |
| Influenza | |
| SARS | |
| Anthrax | |
| Plague | |
| Untreated water | Hepatitis A/E |
| Acute diarrhea | |
| Air travel | Influenza |
| SARS | |
| Cruise ship | Legionella |
| Unprotected sex | HIV |
| Flooding/natural disaster | Leptospirosis |
| Melioidosis | |
| Endemic typhus | |
| Pilgrimage/Hajj | Meningococcus |
| Pregnancy | Hepatitis E |
| Construction | Melioidosis |
| Leptospirosis |
In addition to extensive cardiopulmonary examination, great care should be given to examination of the reticuloendothelial system. In addition, clues to diagnosis can be gleaned from thorough skin examination for both rashes and animal or insect bites, as diseases causing critical illness and dermatological findings are limited.1,7,8,11,15 Table 77-5 outlines the major physical examination findings that are seen with certain disease syndromes in a traveler. But most importantly, travel-related critical illness can be categorized into clinical syndrome, which is essential in the early stages of empiric therapy or when a detailed epidemiologic history is not obtainable. While there is overlap between many of these syndromes and some pathogens are associated with more than one syndrome, we believe this approach to be instrumental in further delineating etiology of critical illness in the traveler. Table 77-6 outlines these clinical syndromes and etiologic agents.1,7,8,11,15
Common Examination Findings of Diseases Associated With Travel-Associated Critical Illness
| Examination Finding | Associated Disease(s) |
|---|---|
| Lymphadenopathy | HIV, rickettsial disease, plague, dengue |
| Hepatomegaly | Malaria, hepatitis A/E, leptospirosis |
| Splenomegaly | Malaria, dengue, trypanosomiasis |
| Jaundice | Malaria, hepatitis A/E, leptospirosis, dengue, HIV, Lassa fever |
| Hemorrhage | Dengue, viral hemorrhagic fevers, meningococcal meningitis, Lassa fever, rickettsial diseases |
| Maculopapular | Dengue, HIV, rickettsial disease, leptospirosis |
| Ecchymosis/petechiae | Rickettsial disease, meningococcal meningitis, viral hemorrhagic fever, leptospirosis |
| Eschar | Rickettsial diseases (scrub typhus), anthrax, African trypanosomiasis, viral hemorrhagic fever |
| Ulcers | Anthrax, plague |
| Urticaria | Schistosomiasis |
Syndromes of Critical Illness and Diseases Associated With Travel-Related Critical Illness
| Clinical Syndrome | Associated Disease(s) |
|---|---|
| Pneumonia/ARDS | Hantavirus, SARS, pneumococcus, influenza, Legionella, plague (pneumonic), melioidosis, Legionella, schistosomiasis, histoplasmosis, coccidioidomycosis, SARS |
| Septic shock/multiorgan system failure | Meningococcal meningitis, viral hemorrhagic fever, dengue hemorrhagic fever, pneumococcus, melioidosis, plague |
| Encephalitis/meningitis | Meningococcal meningitis, dengue, Japanese encephalitis, African trypanosomiasis, rabies, viral hemorrhagic fever |
| Fulminant hepatic failure | Hepatitis A/E |
| Diarrheal illness, hemolytic uremic syndrome | Enterotoxic E coli |
| Necrotizing soft tissue infection | Vibrio fulnificans, MRSA, Streptococcus pyogenes |
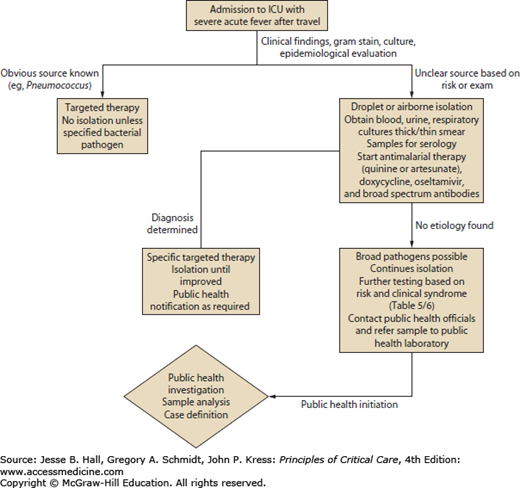
The wide array of diseases seen with worldwide travel makes early diagnosis difficult. Furthermore, many diseases are only diagnosed by serology (hantavirus), or biopsy (tularemia), or specialized culture requirements that make isolation difficult (Y pestis). These diagnostics also require time, which can be difficult given the urgency of treatment of a critically ill patient.16-18 However, by focusing on the most rapidly lethal diseases of a traveler, particularly those with time-sensitive treatment regimens, a systematic approach to quick diagnostics and treatment can be reached. Severe (including cerebral) malaria, meningococcal meningitis and sepsis, dengue fever, viral hemorrhagic fevers (VHF) (eg, Ebola, Marburg), severe coronaviruses (SARS and MERS-CoV), influenza, plague, and tularemia are lethal pathogens causing rapid multiorgan system failure in a traveler and will require rapid recognition for both therapeutic and protective measures, particularly with highly contagious diseases such as viral hemorrhagic fever, influenza, plague, and severe coronaviruses (MERS-CoV).1,6,7,9,11,16-18 Figure 77-1 outlines a stepwise approach to the critically ill traveler. Once recognized, all travelers should undergo respiratory and contact isolation. Due to the high risk of malaria, all critically ill patients in areas endemic for malaria should undergo a thick or thin blood smear with Giemsa or Romanowsky stain. Blood cultures, respiratory cultures, and urine cultures should be obtained in all cases. If signs of meningitis or encephalitis are present, a lumbar puncture should be performed. Addition of a nasal swab for respiratory viruses (especially influenza) is rapidly available in most institutions and should be performed regardless of seasonal variation given the travel history to potential areas of influenza endemicity. Finally, additional blood should be drawn, and stored, for serology for the wide number of pathogens possible.1,6,7,9,11,16-18 For example, VHFs require specialized testing in state and federal laboratories, and thus all samples will need shipment with a time delay before yielding a diagnosis.8 Additional testing based on the risk factors or clinical syndromes outlined in Tables 77-5 and 77-6 should be performed, but results can take time, so the initial testing outlined in Figure 77-1 is essential regardless of clinical syndromes or epidemiologic risk.8
In a critically ill patient with multiorgan failure, ARDS and sepsis will require certain support regardless of etiologic agent. Paramount is the use of a lung-protective ventilation strategy.19,20 Low-tidal-volume ventilation as based on the ARDS Network algorithm should be used in all causes as it has been proven to lower mortality in patients with ARDS. Initial tidal volumes of 6 mL/kg ideal body weight should be employed and lowered if the plateau pressures remain elevated above 30cm H2O. Higher levels of positive end-expiratory pressure (PEEP) should also be employed, particularly if the PaO2/FiO2 remains low.19,20 Other maneuvers or modalities, including prone positioning or nonconventional forms of mechanical ventilation (eg, airway pressure release ventilation), have never been shown to reduce mortality, and thus should be used sparingly.20-22 Other adjuvant therapies for a critically ill traveler with ARDS have been tried as well without consistent success. Steroids and other anti-inflammatory agents have been used in influenza, avian influenza, anthrax, and VHF.23,24 However, this experience has been limited to case reports only and in some cases may be harmful. Other agents, such as immunoglobulin therapy and aerosolized antibiotics have also been employed on a case report basis and cannot be recommended routinely. The management of septic shock should likewise be supportive. Resuscitation with intravenous fluids, colloid, blood, and subsequent vasopressor therapy, and renal replacement therapy should be administered. As with all patients in septic shock, the effect of therapy should be measured closely (eg, central venous catheter, mixed SvO2, lactate, etc).21,23
The role of noninvasive positive pressure ventilation (NPPV) in a traveler with hypoxemic respiratory failure and ARDS is more complicated. In heterogeneous patient populations with acute hypoxemic respiratory failure, NPPV has been shown to reduce the likelihood of endotracheal intubation (57%), ICU length of stay, and mortality in some patient populations (eg, cardiogenic pulmonary edema, obstructive lung disease).25 Regarding ARDS from an infectious agent as the cause for acute hypoxemic respiratory failure, a recent study at experienced NPPV centers showed that early application of NPPV led to improvement in gas exchange, avoidance of intubation, and less associated ventilator-associated pneumonia. However, intubation remained high in patients when illness was more severe (Simplified Acute Physiology Score II >35) or hypoxemia did not improve after 1 hour (PaO2/FiO2 <175), suggesting that NPPV may be useful in early ARDS in less severe patients who respond quickly.21,26 With the SARS experience in Canada, NPPV was also associated with an increased risk of disease transmission. This experience was based largely on case studies and was not reported with SARS cases in Asia. Thus, the likelihood of increased disease transmission with NPPV use in a febrile critically ill traveler can increase disease transmission.23,26-28 In summary, the use of NPPV with an infected ARDS patient remains controversial, with some benefit possible in less severe, early ARDS cases that can potentially increase disease transmission.
Figure 77-1 outlines the approach to a critically ill traveler with initial diagnostics and respiratory isolation. Severe malaria, rickettsial disease with multiorgan failure, and bacterial sepsis with multiorgan failure (meningococcus, plague, tularemia) can respond to early antimicrobial therapy. For viral syndromes such as MERS-CoV, VHF, and dengue, supportive care is essential. For influenza, however, therapy with a neuraminidase inhibitor is essential in the first 48 hours. Therefore, empiric therapy with an antimalarial, specifically artesunate or quinine, should be initiated while diagnostic testing is pending.18,29 The administration of doxycycline for rickettsial disease should occur along with a broad-spectrum antibacterial, such as ceftriaxone, ampicillin-sulbactam, or imipenem.18,29 Finally, for patients traveling to areas of active influenza (winter months), oseltamivir should be initiated.23
SPECIFIC DISEASES AND THERAPY
Malaria is the most classic disease associated with travel and is endemic throughout most of the tropics. Over 243 million will develop symptomatic malaria annually, with most of these cases being attributable to P falciparum (90%), but P vivax and P knowlesi can also cause symptomatic disease.30 Indeed, P vivax, P ovale, and P malariae have been associated with severe disease in some rare cases. Severe malaria is defined as a parasitemia of 5% to 10% of red blood cells (5% in low incidence regions and 10% in high incidence regions) with signs of end organ damage: altered consciousness with or without seizures, respiratory distress or ARDS, hypotension and heart failure, metabolic acidosis, renal failure with hemoglobinuria (“blackwater fever”), hepatic failure, coagulopathy, severe anemia, and hypoglycemia.31 Cerebral malaria with encephalopathy and seizures carries the worst prognosis. Severe malaria requires rapid treatment due to the potential for rapid decline and death within 24 hours of onset, and as such, therapy should be initiated when suspected.31,32
The clinical manifestations of severe malaria vary with age, species, and geography. Young children (ages 2-5 years) and pregnant women are at high risk for severe malaria.31,32 Older children and adults develop partial immunity to febrile malaria episodes (but not to malaria infection) after repeated infection, and thus are at relatively low risk for severe disease. Travelers to areas where malaria is endemic generally have no previous exposure to malaria and thus are at high risk for progression to severe disease, particularly with P falciparum. For this reason, malaria is an extremely important consideration in all travelers with severe disease.
Parenteral therapy is preferred for rapid treatment.31,32 There are two major classes of drugs available by IV administration: the cinchona alkaloids (quinine and quinidine) and the artemisinin derivatives (artesunate, artemether, and artemotil).31-35 Based on clinical trials, artesunate is superior for treatment of severe falciparum malaria when compared to quinine.31-35
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree