(1)
Division of Pulmonary and Critical Care Medicine, Eastern Virginia Medical School, Norfolk, VA, USA
Keywords
PainAgitationDeliriumAnalgesiaPropofolBenzodiazepinesLorazepamMidazolamFentanylMorphineCAM-ICU scoreHaldolDexmedetomidineGlasgow Coma Scale (GCS)Richmond Agitation-Sedation Scale (RASS)Sedation vacationNeuromuscular blocking agents (NMBA)“Today we will be mixing… the Draught of Peace, a potion to calm anxiety and soothe agitation. Be warned: if you are too heavy-handed with the ingredients you will put the drinker into a heavy and sometimes irreversible sleep, so you will need to pay close to what you are doing,” admonished potions teacher Severus Snape as he addressed Harry Potter and his classmates at Hogwarts School of Witchcraft and Wizardry.
From Harry Potter and the Order of Phoenix, JK Rowling
Pain and anxiety are almost universal feature of ICU patients. Clinically significant pain and anxiety have been reported in up to 70 % of ICU patients. Anxiety is often caused or exacerbated by uncontrolled pain. Severe anxiety is not limited to mechanically ventilated patients; indeed Treggiari-Venzi and colleagues demonstrated that up to 30 % of non-intubated SICU patients had severe anxiety [1]. Anxiety has numerous adverse effects, consequently, the control of anxiety is an integral component of the management of the ICU patient. Traditionally, the liberal use of sedatives was recommended in order to treat anxiety, with ventilated patients being heavily sedated with continuous infusions of sedative agents. The traditional approach to sedation in the ICU was one of deep sedation in which the patient was “snowed”. We now know that is approach to harmful and associated with numerous complications. Furthermore, ICU patients can be effectively managed with minimal or no sedation. Indeed, Strom et al. performed a RCT in which 140 mechanically ventilated patients were randomized to standard sedation or no sedation [2]. In this study patients were randomized to receive no sedation or sedation with propofol for 48 h. After 48 h the sedative was change to an infusion of midazolam. Both groups were treated with bolus doses of morphine (2·5 or 5 mg) as required for pain control. In cases in which delirium was suspected, intravenous haloperidol was given as bolus doses. Patients who received no sedation had greater ventilator and ICU free days with no apparent adverse events. This study dispels the common myth that all patients who require mechanical ventilation should receive sedative medications [3].
The primary objective of sedation is too allay anxiety, enhance patient comfort, promote sleep and facilitates mechanical ventilation. The first step in this process is to treat pain (see Fig. 15.1). Prospective studies confirm that the majority of patients who are treated in ICUs have pain, which makes the assessment of pain and provision of adequate analgesia essential components of ICU care [4]. Studies on ICU-discharged but still-hospitalized patients showed that 82 % remembered pain or discomfort associated with the endotracheal tube and 77 % remembered experiencing moderate to severe pain during their ICU stay [5, 6]. The negative physiologic and psychological consequences of unrelieved pain in ICU patients are significant and long-lasting [7]. Pain management is best achieved with boluses of fentanyl (25–50 μg). In patients in whom pain is poorly controlled with bolus doses of analgesia a continue infusion of fentanyl is recommended. Thoracic epidural anesthesia/analgesia be considered for postoperative analgesia in patients undergoing abdominal aortic aneurysm surgery. Likewise, thoracic epidural analgesia should be considered for patients with traumatic rib fractures. Sedative agents should be considered in patients who continue to display anxiety once pain control is achieved. Maintaining light levels of sedation in adult ICU patients is associated with improved clinical outcomes (e.g., shorter duration of mechanical ventilation, shorter ICU length of stay, less delirium). In 251 critically ill patients, Shehabi et al. identified deep sedation within 4 h of commencing ventilation as an independent negative predictor of the time to extubation, hospital death, and 180-day mortality [8]. Similarly, in a study of 259 patients ventilated for greater than 48 h these authors demonstrated that the depth of sedation in the first 48 h was independently associated with longer time to extubation, hospital death and 180-day mortality [9]. A minority of ICU patients have an indication for continuous deep sedation, for reasons such as the treatment of intracranial hypertension, severe respiratory failure, refractory status epilepticus, and prevention of awareness in patients treated with neuromuscular blocking agents
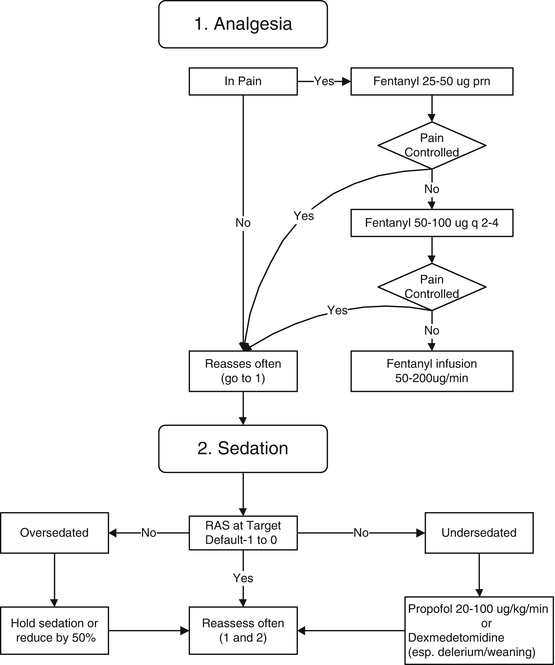

Fig. 15.1
Analgesia/sedation protocol for mechanically ventilated patients. Adapted with Permission from Vanderbilt University, Drs Girard, Pandharipande and Ely
Strategies using nonbenzodiazepine sedatives (either propofol or dexmedetomidine) are preferred over sedation with benzodiazepines. A meta-analysis which compared a benzodiazepine sedative strategy with a nonbenzodiazepine sedative strategy, demonstrated that the nonbenzodiazepine strategy was associated with a shorter ICU length of stay and duration of mechanical ventilation without a mortality difference [10]. Patients who have been receiving prolonged infusions of benzodiazepines at high doses in the acute setting are at risk of withdrawal on discontinuation. In mechanically ventilated ICU patients managed with individualized targeted sedation, Pandharipande et al. demonstrated that the use of a dexmedetomidine infusion resulted in more days alive without delirium or coma and more time at the targeted level of sedation than with a lorazepam infusion [11]. The 28-day mortality in the dexmedetomidine group was 17 vs. 27 % in the lorazepam group (P = 0.18).
Based on these data three sedative strategies are currently recommended:
Propofol infusion titrated to the lowest possible dose
Dexmedetomidine infusion
Bolus doses of lorazepam (1–5 mg)
Infusions of benzodiazepines are best avoided as they are associated with a higher incidence of delirium. None of the sedative agents have analgesic properties, hence opiates are required for pain control. Opiates are also associated with an increased risk of delirium. However, opiates act synergistically with sedative agents allowing lower doses of each agent to achieve the desired effect.
An essential component of the pain, agitation and delirium pathway (PAD) is the regular bedside assessment of pain, agitation and delirium using validated scoring tools [12]. Assessing the degree of sedation and titrating the drug regimen to predetermined end-points is essential as both over sedation and inadequate sedation are associated with significant complications. Complications of under-sedation include severe anxiety with delusional behavior, interference with medical and nursing care, sympathetic over-activity with increased myocardial oxygen consumption, self-injury and self extubation. Severe anxiety with inadequate sedation has been reported to be the most important factor leading to unplanned extubations. Oversedation is associated with significant morbidity including prolonged intubation with an increased risk of pulmonary complications and disorientation and delirium. In addition, oversedation may mask significant neurological and neuromuscular complications.
In anxious patients it is important to exclude treatable causes of anxiety and not just increase the amount of sedative drugs being used. Treatable causes of anxiety include:
uncontrolled pain (NB)
ventilator settings inappropriate (esp inadequate flow rate)—respiratory incoordination
drug or alcohol withdrawal syndrome,
increased work breathing, e.g. pneumothorax, kinked/blocked tube
pulmonary edema
Loud ventilator alarms and monitors
Poor communication with patient as regards diagnosis, therapy etc.
“Doctor, doctor, my patient is very agitated!”
What is your next step?
a.
Give 2 mg Ativan to the nurse
b.
Give 5 mg Haldol to the patient
c.
Take 5 mg morphine for yourself
d.
Look at your patient.
Assessing the Level of Pain and Sedation
A patient’s self-report of pain is considered the “gold standard,” and clinicians should always attempt to have a patient rate his or her own pain first [12]. Chanques and colleagues demonstrated that a 0–10 visually enlarged horizontal numeric rating scale was the most valid and feasible of five pain intensity rating scales tested in over 100 ICU patients [13]. The Behavioral Pain Scale (BPS) and the Critical-Care Pain Observation Tool (CPOT) are the most valid and reliable behavioral pain scales for monitoring pain in medical, postoperative, or trauma adult ICU patients who are unable to self-report and in whom motor function is intact and behaviors are observable [12, 14–17]. Vital signs (or observational pain scales that include vital signs) should not be used alone for pain assessment in adult ICU patients.
Ongoing clinical evaluation is the most effective method of assessing sedation. In order to provide a more consistent and objective means of assessing the degree of sedation a number of sedation scales have been developed. Reliable sedation scales can enhance communication among caregivers, improve consistency in drug administration, be used in sedation protocols and improve precision of medication titration as patient needs change over time. The routine use of a sedation scale, including frequent adjustments of the sedation target as needed, is strongly endorsed by evidence-based guidelines [12].
The Glasgow Coma Scale (GCS) was developed to assess the level of consciousness of trauma patients. This scale is commonly used in neurosurgical ICU’s. The GCS is however essentially a measure of pathologic obtundation and cannot be recommended for monitoring the level of sedation in ICU patients. The Ramsay Sedation Scale and variations of this scale are frequently used method of assessing and documenting sedation in the ICU. The Ramsay scale was reported by Ramsey and colleagues in 1974 in a study which assessed the use of alphaxalone-alphadolone (Althesin) in 30 ICU patients [18]. The Ramsey scale has a number of significant limitations when used to assess the level of sedation in ICU patients and is not recommended
The Ramsey Sedation Scale
I.
Anxious and agitated
II.
Cooperative, orientated and tranquil
III.
Drowsy, responds to verbal commands
IV.
Asleep, responds briskly to light stimulation
V.
Asleep, sluggish response to stimulation
VI.
Asleep, no response to stimulation
Over 25 instruments have been developed to measure consciousness in the ICU [18, 19]. The Richmond Agitation-Sedation Scale (RASS) which was specifically designed to assess sedation in the ICU, is that scale which has been most extensively tested for reliability and validity in adult ICU patients and is currently recommended as the sedation scale of choice [20, 21]. RASS is a 10-point scale, with four levels of anxiety or agitation (+1 to +4 [combative]), one level to denote a calm and alert state (0), and 5 levels of sedation (−1 to −5) culminating in unarousable (−5).
The Richmond Agitation-Sedation Scale (RASS)
+4 Combative
+3 Very agitated
+2 Agitated
+1 Restless
0 Alert and calm
−1 Drowsy
−2 Light sedation
−3 Moderate sedation
−4 Deep sedation
−5 Unarousable
Sedation Vacations
Observational and randomized trials have demonstrated that protocols directed at minimizing the use of sedative infusions shorten the weaning process. Specifically, approaches intended to avoid over-sedation by limiting the use of continuous infusions either through sedation assessment scoring or by daily cessation of sedation, decreases duration of mechanical ventilation and duration of ICU stay [22–24].
Girard et al. published the results of a trial that employed a “wake up and breathe” strategy (the ABC trial) [25]. Patients randomized to a daily awakening trial followed by a SBT (versus SBT alone) experienced increased time off of mechanical ventilation, decreased time in coma, decreased ICU and hospital length of stay and improved survival at 1 year. Based on this data, the sedation should be stopped (or the dose significantly reduced) each morning; this allows for neurological assessment of the patient, performance of a SBT, reassessment of the goals of sedation, and an individualized exercise/occupational program. Schweickert and colleagues performed a RCT in which patients who remained ventilator dependant for more than 3 days were randomized to early physical and occupational therapy which was coupled with daily awakenings [26]. Patients in the intervention group had shorter duration of delirium and more ventilator-free days with significantly more patients returning to an independent functional status.
Non-pharmacologic Interventions
Minimize sleep deprivation related to noise and light
Establish a “normal” day-night cycle
Orient the patient (place, day, time) as frequently as possible
Communicate goals of treatment with patient (if possible)
Music therapy
Ensure comfort by turning and positioning
Ensure ventilator synchrony
Delirium
Delirium is characterized by a disturbance of consciousness with accompanying change in cognition. Delirium typically manifests as a constellation of symptoms with an acute onset and a fluctuating course. Delirium in the ICU is very common, the incidence ranging from 45 to 87 %. The incidence appears to vary according to whether the studied population is composed exclusively of mechanically ventilated patients. The symptoms of delirium have been organized into cognitive and behavioral groups. Common cognitive symptoms include disorientation, inability to sustain attention, impaired short-term memory, impaired visuospatial ability, reduced level of consciousness, and perseveration. Common behavioral symptoms include sleep-wake cycle disturbance, irritability, hallucinations, and delusions. The manifestations of delirium can vary widely among patients. While some patients may manifest somnolence and even coma, others appear anxious, disruptive, or combative. Recognition of this symptom variability has led to the classification of delirium into motoric subtypes. One such subtype is hyperactive delirium, of which the manifestations include agitation, hypervigilance, irritability, lack of concentration, and perseveration. One the other hand, hypoactive delirium manifests as diminished alertness, absence of or slowed speech, hypokinesia, and lethargy. Mixed delirium, as the name implies, includes manifestations of both hyperactive and hypoactive delirium. The mixed and hypoactive forms of delirium are the most common in the ICU. Hypoactive delirium tends to occur more frequently in older patients and carries worse prognosis. While multiple clinical risk factors have been identified and numerous pathophysiologic pathways have been hypothesized, the pathophysiology of delirium remains poorly understood [27]. Studies using magnetic resonance imaging have shown a positive association between the duration of delirium in the ICU and both cerebral atrophy and cerebral white-matter disruption [28, 29]. These preliminary investigations indicate either that delirium in the ICU gives rise to alterations in brain structure or that the presence of such cerebral atrophy and white-matter disruption renders patients more susceptible to delirium. The major predisposing factors include respiratory failure, older age, alcohol abuse, dementia and medications. Classes of medications commonly associated with delirium include anticholinergic agents, benzodiazepines, and opiates. Benzodiazepines appear to be strongly associated with delirium in ICU patients [11].
Delirium is associated with increased ICU and hospital length of stay. The presence of delirium has important prognostic implications; in mechanically ventilated patients it is associated with a 2.5 fold increase in short-term mortality and a 3.2 fold increase in 6-month mortality. Furthermore, delirium is associated with the development of post-ICU cognitive impairment. Delirium is frequently undiagnosed unless specific diagnostic instruments are used. Therefore routine monitoring of delirium in all ICU patients is recommended [12]. The Confusion Assessment Method for the ICU (CAM-ICU) and the Intensive Care Delirium Screening Checklist (ICDSC) are the most valid and reliable delirium monitoring tools (see Fig. 15.2) [30]. Gusmao-Flores et al. performed a metaanalysis comparing the CAM-ICU with the ICDSC [31]. The CAM-ICU had a high sensitivity and specificity with a diagnostic odds ratio of 103.3 and a ROC of 0.97. The ICDSC has moderate sensitivity and good specificity (ROC 0.89). The available data suggest that both CAM-ICU and the ICDSC can be used as a screening tool for the diagnosis of delirium in critically ill patients. It is however not clear that the use of these scales is more sensitive than unstructured assessments made by trained bedside nurses who are prompted to look for delirium [32].
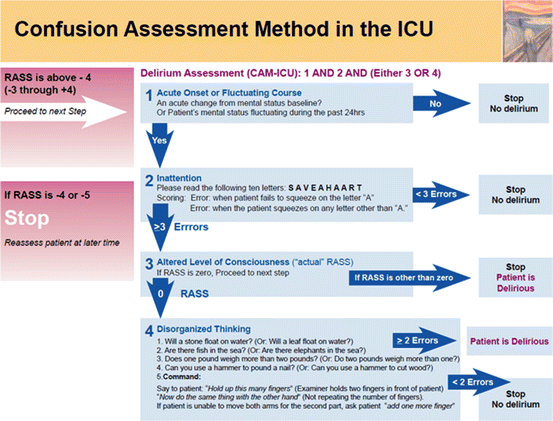

Fig. 15.2
Confusion assessment method for the ICU (CAM-ICU)
Non-pharmacological approaches such as physical and occupational therapy decrease the duration of delirium and should be encouraged. The use of pharmacologic agents to prevent delirium is not recommended, as there is no compelling data to demonstrate that this reduces the incidence or duration of delirium. Page and colleagues randomized 141 mechanically ventilated patients to receive haloperidol 2·5 mg or placebo intravenously every 8 h, irrespective of coma or delirium status [33]. Patients in the haloperidol group spent the same number of days alive, without delirium, and without coma as did patients in the placebo group.
Traditionally haloperidol has been used to treat delirium in the ICU. There is however no published evidence that treatment with haloperidol reduces the duration of delirium. Girard et al. randomized 101 mechanically ventilated patients with delirium/coma to receive haloperidol, ziprasidone, or placebo every 6 h for up to 14 days [34]. Treatment with antipsychotics did not improve the number of days alive without delirium or coma, nor did it increase adverse outcomes. Devlin et al. performed a double-blind, placebo controlled, multicenter study which evaluated the efficacy and safety of quetiapine in 36 ICU patients diagnosed with delirium [35]. Rescue intravenous haloperidol was allowed to be used in both groups. The results showed that quetiapine was associated with quicker resolution of delirium, reduced time of delirium and agitation, and reduced haloperidol requirement compared to placebo. There was however no difference in the duration of ICU stay, length of hospitalization, and hospital mortality. Two clinical trials have found that antipsychotics, compared with placebo, hasten the resolution of delirium in hospitalized patients without critical illness. Hu et al. found that haloperidol and olanzapine each led to earlier improvements in Delirium Rating Scales scores among 175 elderly patients, compared with placebo [36]. Similarly, Kalisvaart et al. found that haloperidol reduced the severity and duration of delirium among elderly patients undergoing hip surgery, compared with placebo [37]. These data suggest haloperidol has a limited role in the treatment of delirium. Atypical antipsychotics may reduce the duration of delirium (see Table 15.1). Dexmedetomidine has been demonstrated to reduce the duration of delirium and may be the agent of choice in these patients [38].
Table 15.1




Dosages and side effects of the atypical antipsychotics
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





