1. Both the parent’s and the anesthesiologist’s interactions with the child during the preoperative visit and induction have significant impact on the child’s postoperative behavior. Distraction, humor, and reinterpretation of medical events are most effective, while empathy and reassurance reinforce maladaptive responses.
2. Most children can be induced by mask unless otherwise contraindicated. A gentle, gradual approach with the mask is preferable to forcing the mask on the face. Even adolescents can be given a choice of induction technique.
3. The incidence of adverse hemodynamic events during induction have diminished since sevoflurane replaced halothane for induction in the United States, but careful observation in the potentially hypovolemic child is still critical.
4. In children with adenotonsillar hypertrophy who obstruct during induction, jaw thrust is the most effective maneuver.
5. Cuffed endotracheal tubes are now available in pediatric-specific designs and can be used safely in place of uncuffed tubes. Proper fit remains critical. Supraglottic airway (SGA) devices are also effective in most children in appropriate circumstances.
6. A thorough understanding of the patient’s underlying condition and the operative requirements dictate the maintenance technique.
7. Neuromuscular blockade (NMB) is often not needed in children and used less frequently than in adult anesthetics.
8. Infants and young children are more sensitive to the adverse respiratory effects of anesthetics, including decreased drive, instability of the rib cage, and atelectasis. They breathe closer to their closing volume, and depend more on diaphragmatic activity and less on intercostal breathing. This must be taken into account when managing intraoperative ventilation.
9. Deep extubation is a good option except when the airway is unstable during induction, when a full stomach condition exists, or when the postanesthesia care unit (PACU) staff is not skilled in airway maintenance.
I. Premedication and patient/family preparation
A. The management of anesthesia in children begins with the preoperative visit, where the anesthesiologist first has the opportunity to establish a rapport with the child and family. In this brief time, the clinician can learn much about the child’s temperament and reaction to strangers, in addition to obtaining the history and performing an examination. This aids in judging whether premedication is indicated and what the family’s expectations and concerns are about the upcoming anesthetic. The conduct of this interaction can create an atmosphere of trust and comfort that the anesthesiologist can use to ease the transition to induction. This is the primary opportunity to establish a relationship with the child, an ability that is one of the paramount skills of the pediatric anesthesiologist. The anesthesiologist must not converse solely with the parents during this critical encounter but should also focus attention on the patient, which can be successful even in preverbal children and serves to set both child and parents at ease. Magic tricks, games, hypnosis, and simple age-appropriate conversation and engagement with the child are all valuable tools. Developmental issues should be explored so that induction and postanesthesia recovery management are appropriate for the child’s developmental age.
B. Induction of anesthesia in infants and children is generally a more sensitive process than in the adult. Sleep disturbances, behavioral regression, and maladaptive behaviors commonly occur after stormy inductions in children, can persist for weeks after surgery, and are highly disturbing to parents (1).
1. The character of interactions between anesthesiologist and child, and parent and child, can be a major influence on the behavior during induction and emergence from anesthesia. Surprisingly, empathy and reassurance by caregivers both promote distress and diminish coping in verbal children, while distraction, humor, and reinterpretation of medical events by the physician are beneficial (2). These positive behaviors can be trained and promoted, and similar findings have been demonstrated in the PACU (3,4).
CLINICAL PEARL When first meeting the family and child, instead of launching into the medical history, interact with the child first, engaging her with questions about school, friends, favorite games, toys, or shows.
2. Premedication with midazolam had been shown to reduce the incidence of maladaptive postoperative behavior (5). The child older than 6 to 9 months who exhibits anxiety that is not improved with behavioral techniques is probably best premedicated (6). Parents’ assessment of the need for premedication may not be reliable—data suggest that they are able to predict uncooperative behavior far better than cooperative behavior. Midazolam (0.25 to 0.5 mg/kg) can be administered orally 10 to 20 minutes before induction of anesthesia. The lower dose has been found as effective as higher doses in producing anxiolysis and cooperation with inhalation induction as long as adequate time is allowed for the drug to take effect (7). Premedication has also been found to be more effective than either parental presence at induction or preoperative teaching programs (8). Preoperative teaching programs have been shown to have beneficial effects that are limited to the preoperative period, and do not appear to carry over to induction or emergence, although one study showed decreased behavioral disturbances 2 weeks after surgery (9,10).
CLINICAL PEARL Premedication may be especially helpful for children who have frequent repeated visits to the operating room (OR) and exhibit any signs of anxiety. Be sure to allow enough time for the drug to take effect.
3. Premedication and induction for the developmentally delayed child should be managed according to the developmental, not chronologic age. A maximum dose of 15 to 20 mg of oral midazolam is usually recommended. Developmentally impaired older large children who are uncooperative may be better managed with a mixture of midazolam and ketamine (0.5 mg/kg and 3 mg/kg, respectively) as midazolam alone may produce disinhibition in some of these patients (11). Glycopyrrolate (0.05 to 0.1 mg/kg) can be added to the mixture as an antisialagogue. For older children and adolescents who are undergoing long procedures such as spinal fusion and instrumentation, the author uses diazepam, 0.1 mg per kg. Intranasal midazolam has been used (0.2 mg/kg) but has the significant disadvantages of burning on instillation and, if the drug drips down into the mouth, having a severely bitter, unpleasant taste (12). It may be reserved as an option for the child who refuses to take the oral preparation. Intranasal dexmedetomidine (1 to 2 µg/kg) is highly bioavailable and effective, inducing a state of somnolence and cooperation, is not painful or unpleasant, but is not anti-anxiolytic per se, and may produce prolonged sleepiness in recovery (13,14).
4. Parental presence during anesthetic induction has become very common for children older than 1 year. The advantages claimed include comfort for the child, the avoidance of separation anxiety, and increased parental satisfaction. Most parents, when properly prepared for what to expect and advised on the most effective behaviors, can be beneficial assistants to their children during induction, and although many report finding induction a stressful experience for themselves, most believe that it is valuable for their child. Parental presence has been shown to be less effective than premedication for anxiolysis, but may contribute significantly to parents’ perceived satisfaction with the perioperative experience (8). Parents must understand that they will be escorted out of the OR as soon as their child loses consciousness, or if a complication develops during induction, so that the anesthesia team can pay full attention to the care of their child without distractions. There should be an assistant whose task is to “take care of the parents” during this period.
II. Induction of anesthesia. In the absence of a full stomach or other contraindication, inhalation induction is most commonly used in infants and younger children. When not contraindicated, older children can be given their choice of induction technique.
A. Inhalation induction is the most common method of induction for pediatric patients without intravenous access. All children have received injections for immunization, and fear of needles is nearly ubiquitous. This single issue creates more anxiety in toddlers and older children than almost any other in the preoperative period, and the avoidance of needles speeds and eases induction in most children. Much anguish can be immediately dissipated once the child learns that there will be “no needles” and that nothing will hurt during induction. Inhalation induction is painless and relatively quick, but having the mask placed on the face may be frightening and unpleasant for some children, so avoid forcing the mask on the child’s face.
CLINICAL PEARL In the mask-aversive child, hold the mask—or even just the circuit cupped in the anesthesiologist’s hand—over the face or under the chin without contact until the child begins to become drowsy. As soon as consciousness is lost, the mask can be applied snugly to create a seal, the anesthetic deepened, and an intravenous line established.
B. Anesthesia can be induced with the child in any position—lying down on the OR table, sitting up, or sitting in the parent’s lap. It is almost always counterproductive to force a child to lie down if he or she does not want to do so. It is important for the anesthesiologist to be completely engaged with the child during inhalation induction and to eliminate other distracting activity (especially noise, preparations, and talking by OR personnel) during this period. It is best if only one person talks continuously to the child in a calm, quiet, and monotonous but reassuring voice. Telling a story to the child, perhaps one that incorporates the events during induction, is beneficial. One may have the child pretend he or she is the pilot of a jet plane or spaceship, and use the mask and the scent of the anesthetic vapor as cues in the story. Other children may be better relaxed by distractive techniques akin to hypnosis. The skilled pediatric anesthesiologist will pick up clues from both parent and child during the preoperative visit as to what is the best approach for each child and adjust the technique accordingly.
C. In the United States, sevoflurane is the primary agent used for inhalation induction. It is nonpungent and does not cause airway irritation. The strong odor of the agent can be partly obscured by using scented oils or flavors in the face mask to increase acceptance. Many pediatric anesthesiologists like to begin with 60% to 70% nitrous oxide (N2O) first and then add sevoflurane once an effect of the N2O is noted. This permits the child to become sleepy before noticing the smell of sevoflurane. There is no need to titrate up the inhaled concentration, as was done with halothane. One can increase the concentration rapidly to 8% sevoflurane (overpressure) (15).
D. In the child with normal cardiac and respiratory function, consciousness is lost within a minute. Induction time is prolonged in children with right to left cardiac shunts.
E. Parents, when present for induction, must be cautioned that when the child goes through stage II he or she may exhibit involuntary movements for a brief period and may develop noisy breathing as hypopharyngeal muscle tone becomes diminished, and that these are normal events. Similarly, if the child loses consciousness with the eyes open, the disconjugate gaze may disturb the parents, and they should be warned and reassured about this event in advance.
F. Sevoflurane may cause myoclonus during stage II of induction, and epileptiform activity has been reported on electroencephalogram (EEG) (16). The significance of this finding is unknown, but hyperdynamic heart rate and blood pressure changes during induction may be associated with this EEG activity, which is more common at minimum alveolar concentration (MAC) >1.5 and is also more common with rapid increases in anesthetic depth such as occurs with induction (17,18).
1. Sevoflurane appears to cause less hypotension at induction than does halothane, primarily due to less decrement of myocardial contractility, although a reduction of peripheral vascular tone still occurs. During the early stages of induction, heart rate and blood pressure increase, especially as compared with halothane (19). The Pediatric Perioperative Cardiac Arrest Registry has seen a decrease in the number of arrests occurring during induction since the widespread replacement of halothane with sevoflurane in the United States (20). Nevertheless, systolic blood pressure is reduced in infants and children at 1 MAC of sevoflurane anesthesia before incision, and one must remain vigilant for reduced cardiac output, especially in infants and children who have prolonged preoperative fasting periods, or who are at risk for myocardial depression (21). The latter group of patients may be better served by alternative induction methods or the early use of adjunctive agents to reduce the concentration of sevoflurane required. Spontaneous ventilation has an advantage in the relatively volume-depleted child, as venous return is enhanced, maintaining filling of the right heart, rather than impeded, as occurs with positive pressure ventilation. Once intravenous access is established, a fluid bolus of 10 mL per kg can be administered safely and rapidly in most children to help counteract the effects of the vapor on cardiac output and peripheral vascular tone that produce a fall in blood pressure.
2. The ease of maintenance of spontaneous ventilation is a virtue of inhalation inductions, although sevoflurane does suppress respiratory drive and weaken respiratory muscle function more than halothane (22). While spontaneous ventilation may be preserved using intravenous induction with the judicious choice of drugs and doses as well, one may more readily back off and decrease the depth of anesthesia with inhalation anesthesia if airway problems ensue, making this technique commonly the one of choice for induction in the patient with a difficult airway.
3. Monitoring during the initiation of inhalation induction can be as rudimentary as a pulse oximeter, end-tidal gas analyzer, precordial stethoscope, and vigilant observation, with the remainder of the standard monitors (electrocardiogram [ECG], blood pressure, temperature) placed as soon as consciousness begins to be lost and the child settles down. Invasive monitoring is placed after induction.
CLINICAL PEARL Attempts to place the complete array of monitors on an anxious child or very active infant usually results in increased anxiety or activity coupled with a monitor screen displaying a jumble of useless motion artifacts. Start with just an oximeter, precordial stethoscope, or, in the most uncooperative child, close observation, and place the monitors expeditiously as soon as he or she stops moving.
4. During induction, especially during stage II, the airway may become partly obstructed as tone in the hypopharyngeal musculature diminishes and the tongue falls back onto the palate and pharynx. Paradoxical chest wall motion may develop due to partial supraglottic obstruction coupled with decreased intercostal muscle function and the relative maintenance of diaphragmatic muscle strength. Careful observation of the pattern of breathing and auscultation with a precordial stethoscope (unfortunately greatly underused today) are the best tools for early detection and diagnosis. Auscultation provides clues to the anatomic origin of airway problems at this stage—higher-pitched inspiratory sounds are more likely to originate at the vocal cords (early laryngospasm), whereas lower-pitched ones are more likely to originate in the SGA (upper airway obstruction due to the tongue or the hypopharynx); expiratory sounds are indicative of wheezing or bronchospasm.
CLINICAL PEARL Recognition of the anatomic location of obstruction is critical both because the effective interventions are different and because the wrong intervention can exacerbate the problem. For example, misdiagnosis of early laryngospasm during a light plane of anesthesia as soft tissue obstruction might prompt the placement of an oropharyngeal airway, a maneuver likely to worsen laryngospasm.
In most cases, the use of augmented, rather than controlled, ventilation coupled with continuous positive airway pressure (CPAP) can attenuate or ameliorate SGA obstruction. An oropharyngeal or nasopharyngeal airway may be helpful. In children with adenoid or tonsillar hypertrophy, who commonly have a high incidence of airway obstruction, jaw thrust was found to be the most effective maneuver to open the airway, as compared with CPAP or chin lift (23). Lateral positioning, which is used more commonly during recovery than induction, has also been shown to be of significant benefit (24).
G. Intravenous induction is utilized when an intravenous line is present, when rapid sequence induction is necessary (full stomach), or when preferred by the patient, as may be the case in some older children or children with mask aversion. The pain of intravenous cannulation can be reduced with the use of local anesthetic, injected intradermally with a 30-gauge needle, applied transdermally with one of the variety of local anesthetics such as eutectic mixture of local anesthetics (EMLA), Ametop, or using gas propelled needleless systems to disperse the local anesthetic through the skin (25). It should be noted that although local anesthesia may eliminate the pain of the intravenous catheter placement, it does not eliminate the fear of needles, and an additional means of distraction should be used to reduce apprehension and enhance cooperation.
1. The dose of most intravenous induction agents is greater in children than in adults. Propofol (3.5 to 4 mg/kg) is most commonly employed, and may be used in combination with an opioid such as fentanyl or remifentanil. Propofol causes burning at the site of injection, which may be attenuated, although not completely eliminated, by the injection of lidocaine with a tourniquet placed proximally to the intravenous cannula (in effect, a Bier block). Thiopental (5 to 6 mg/kg) does not cause pain with injection, but its long elimination half-life can result in prolonged lethargy and after effects that last for hours after emergence. This drug is no longer available in the United States. Etomidate (1 to 2 mg/kg) may be used in children with hemodynamic instability. As in adults, any drug that decreases sympathetic drive can cause hypotension in the hypovolemic or hemodynamically compromised child, and intravascular volume should be repleted before induction and drug doses should be reduced and given incrementally when indicated.
2. Rapid sequence induction can be performed when there is a full stomach or risk of aspiration of gastric contents. Cricoid pressure is of questionable efficacy in preventing regurgitation of gastric contents in both infants and children, and may distort the laryngoscopic view (26).
a. Rapid sequence induction is perhaps the last remaining indication for the use of succinylcholine in pediatric anesthetic practice. Concerns over the risk of bradycardia (which can be prevented with the pretreatment with atropine) and the potentially catastrophic precipitation of a hyperkalemic response in a child with a previously undiagnosed myopathy or malignant hyperthermia have prompted many pediatric anesthesiologists to employ an intermediate-acting nondepolarizer such as rocuronium (0.75 to 1 mg/kg) instead. Some pediatric anesthesiologists use a “modified rapid sequence induction,” that is, without the use of a muscle relaxant, substituting instead a rapid-acting opioid such as remifentanil (1 µg/kg with sevoflurane, 2 µg/kg with propofol). This has been shown to produce comparable intubating conditions in about the same time as the use of a muscle relaxant (27,28).
H. The airway may or may not be secured, depending on (i) the age of the child, (ii) the stability of the natural airway, (iii) aspiration risks, and (iv) the demands of the surgical procedure.
1. The use of the face mask for airway maintenance is usually restricted to short cases. Patients with increased airway reactivity may particularly benefit from this approach, as studies in children with upper respiratory infections have shown that this produces the least irritation of the airway (29). When the surgical procedure is very brief, for example, myringotomy and ventilation tube placement, general inhalation anesthesia through face mask may eliminate the need to start an intravenous line. This requires meticulous and continuous attention, as the head will be repositioned from side to side to facilitate surgical access to the auditory canal.
CLINICAL PEARL The use of a precordial stethoscope, coupled with observation of chest wall motion and the capnographic trace, will help ensure that the anesthesiologist maintains the patient’s airway patency and is aware of early signs of obstruction.
It is most important that the anesthesiologist never exerts pressure on the submental space when holding the mask in place. This will push the tongue up toward the palate and lead to upper airway obstruction.
2. SGA devices have gained enormous popularity in recent years and are manufactured in an array of pediatric sizes. They are especially useful for short- or moderate-length procedures where spontaneous or augmented ventilation is planned. SGA devices are tolerated at lighter levels of anesthesia than an endotracheal tube, and produce less airway irritability when used in children with recent upper respiratory illness (29,30). By virtue of their larger caliber, they introduce less airway resistance than an endotracheal tube, and therefore decrease the work of breathing during spontaneous ventilation under anesthesia (31). Although these airway devices can be used with controlled positive pressure ventilation, they may be less advantageous than an endotracheal tube for this mode of ventilation, especially in smaller children. They tend to leak at lower airway pressures and cannot produce a leakless seal like a cuffed endotracheal tube. They may also be more prone to displacement with alterations in head position. Some devices may produce more gastric distension than an endotracheal tube, although newer devices incorporating a conduit to the esophageal inlet may help prevent this. Displacement of and excessive leak around the SGA can occur from cuff overinflation and with the use of N2O (32,33).
CLINICAL PEARL The base of the tongue may impede passage of some SGAs in the pharynx of small children. This can be bypassed by inserting it partly inflated sideways alongside the tongue with the bowl facing the midline (see Fig. 7.1). Once the device passes beyond the base of the tongue, it is centered in the mouth and advanced into final position as in the adult, and inflated.
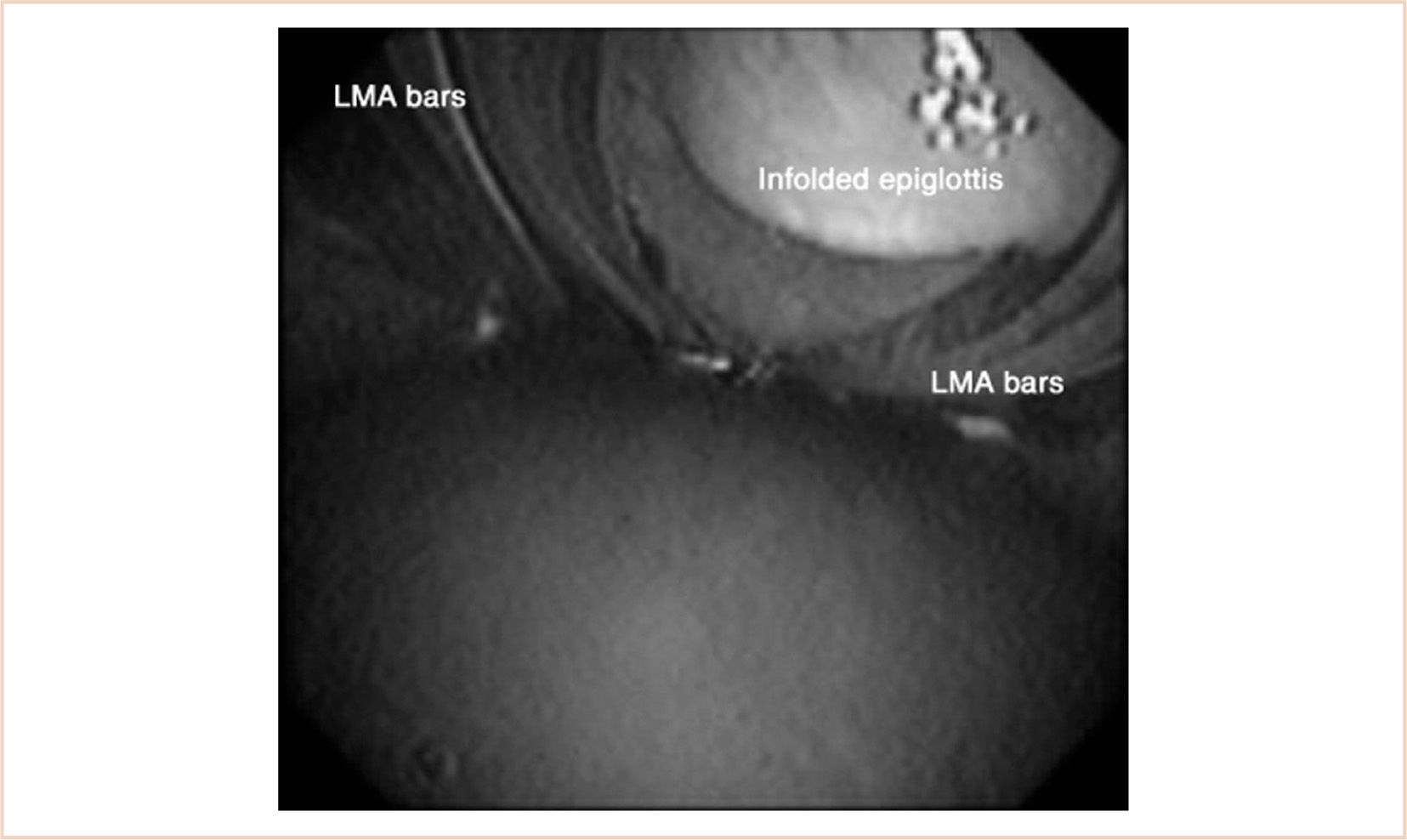
Another problem is that the epiglottis is often folded into the bowl in children under 10 kg (34). This happens in at least 30% of patients with the laryngeal mask airway (LMA), and although airway obstruction is relatively uncommon, anything less than a clear airway should alert the anesthesiologist to the potential for malposition of the device. This problem has also been noted with other devices (35) (see Fig. 7.2). Sealing pressure does not appear to be a reliable sign of proper position in infants and children (36). In addition, several investigators have reported a higher incidence of airway complications in infants using an SGA, including laryngospasm, secretions, hypoxemia, and obstruction; overall, the failure rate appears to be less than 1% (37,38).
3. The endotracheal tube remains the “gold standard” for securing the airway. It affords a dependable airway under most circumstances, is usually easy to insert, permits the use of positive pressure ventilation and positive end-expiratory pressure (PEEP), and can minimize the risk of aspiration. Tube position is especially critical in pediatric patients, as relatively small changes in head and body position may displace the tube. Flexion of the neck tends to advance the tube deeper in the trachea, risking endobronchial intubation, while extension or rotation of the neck risks extubation because of cephalad displacement (39). The former effect is of greater magnitude.
4. For many years, the use of cuffed endotracheal tubes was discouraged in children younger than 6 to 8 years. Uncuffed tubes, sized to produce a leak pressure of 18 to 30 cm H2O, were used instead. There were several reasons for this. The pediatric subglottic airway provides a natural seal with a properly sized uncuffed tube. Cuffed tubes, when overinflated, can produce a reduction in tracheal epithelial blood flow, causing injury, edema, and postintubation stridor, or if prolonged, subglottic stenosis. The design of small, cuffed tubes was often poor, and optimal positioning was difficult due to the excessive length of the cuff (40,41). A poorly designed cuff shape may exert excessive pressure even when not obviously overinflated, because transmural pressure can be unevenly distributed if the contact point with the tracheal wall is only at the very center of the cuff (a football-shaped rather than a sausage-shaped cuff). As the tubing had to be increased in thickness to accommodate the channel to the pilot balloon as well as the space taken up by the cuff itself, when sizes smaller than 4.5 tubes were inserted, it was often necessary to downsize by one half size. Most of these problems have been ameliorated by contemporary tube design, and the use of cuffed endotracheal tubes is increasing in pediatric practice. The Microcuff (Halyard Health, Inc.) tube not only seals with lower transmural pressure, but also its thin wall takes up no additional caliber and it may be more effective at preventing aspiration around the cuff (42–44) (see Fig. 7.3).
CLINICAL PEARL The appropriate endotracheal tube size in children over a year of age usually is equal to the child’s height in centimeter divided by 20.
5. Nasal intubation is indicated when the surgical procedure will be hampered by the presence of an oral endotracheal tube, such as in dental or oral surgery, or when access to the airway through the mouth is impeded by underlying conditions of the patient, as may occur in trauma or congenital or acquired conditions limiting mouth opening. Because adenoid hypertrophy is common in children, blind nasal intubation is generally contraindicated, and one must confirm that the tip of the tube is clear of any adenoidal tissue before introducing it into the trachea.
CLINICAL PEARL Placing the tip of the endotracheal tube in the flange of a red rubber catheter and passing it through the nose into the hypopharynx or placing a suction catheter in the endotracheal tube as an obdurator prior to passage through the nose can prevent the impaction of blood, secretions, or adenoid in the tip of the tube.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree