Fig. 14.1
Classification scheme for choledocholithiasis. Primary gallbladder stones form de novo within the biliary tree. In secondary choledocholithiasis stones form in the gallbladder and migrate through the cystic duct into the biliary tree
Primary stones represent only 5 % of cases of choledocholithiasis in the United States. However, they are an important cause of choledocholithiasis in Southeast Asian nations and in patients with biliary tree pathology [10–12]. These stones typically form from bilirubin, and can be either intrahepatic or extrahepatic.
Extrahepatic primary bile duct stones are known as brown stones and are the most common type of primary bile duct stones. They are composed of a combination of fatty acids, calcium bile salts, and cholesterol [13]. Brown stones are found most commonly in Asian populations and their pathogenesis is thought to be due to a combination of bile stasis and bacterial infection. Bile duct stasis is usually due to obstruction from strictures, foreign bodies, or papillary stenosis. Obstruction in turn leads to bacterial overgrowth and production of bacterial β-glucuronidase, deconjugating bilirubin and forming insoluble calcium bilirubinate [11, 14–16]. Escherichia coli, Bacteroides spp. and Clostridium spp. are commonly isolated organisms. The fact that brown stones are more common in rural Asian populations and that this prevalence recedes upon emigration to Western countries suggests that their formation involves a dietary or environmental component [11, 15]. Protein-deficient animals have reduced levels of β-glucuronidase inhibitors in their bile, which has led to the hypothesis that the low protein diets of rural Asians contribute to their higher prevalence of brown stones [15]. Furthermore, the presence of periampullary diverticula has been shown to significantly increase the risk of developing brown bile duct stones [17]. Finally, CBD dilation has frequently been associated with the development of primary biliary stones after cholecystectomy [18]. As such, cholecystectomy at a young age is a risk factor for the development of primary stones [19].
Intrahepatic primary stones can be black mixed cholesterol or pure cholesterol [18]; the black mixed cholesterol type is more common. These CT hyperdense stones have a black outer layer of calcium bilirubinate over a core composed of up to 50 % cholesterol [11, 12]. The pathogenesis of these stones is not well understood but bacterial infection is thought to contribute.
Pure cholesterol intrahepatic stones are similar in composition to those found in the gallbladder, however their pathogenesis is thought to be different, as many of these patients do not have cholelithiasis [11, 20]. Deficiencies in antinucleating factors have been hypothesized as one of the causes for intrahepatic stones. These factors, which normally counteract cholesterol nucleating factors, slow crystal precipitation and stone formation [21].
Secondary stones are formed in the gallbladder and subsequently migrate into the biliary tree. In the Western World, they are a far more common cause of choledocholithiasis as compared to primary stones. Secondary stones are typically mixed stones, composed primarily of cholesterol with a pigmented shell and about 80 percent of all stones found in the gallbladder fall into this category [11]. Recent research has shown that the pathogenesis of these stones involves multiple concurrent factors including cholesterol supersaturation in bile, crystal nucleation, gallbladder dysmotility, and gallbladder absorption and secretion abnormalities [22]. Most secondary stones remain in the gallbladder. However, approximately 10 % of stones will migrate from the gallbladder into the biliary tree to become symptomatic [23]. The fate of these stones determines both symptomatology and outcomes of secondary choledocholithiasis.
Presentation and Diagnosis
Regardless of origin (primary vs. secondary), gallstones in the biliary system become symptomatic when they obstruct the outflow of bile and/or pancreatic secretions. Consensus thinking holds that most gallstones that exit the cystic duct pass asymptomatically into the duodenum. Furthermore, most stones that remain in the CBD obstruct the flow of biliopancreatic fluid only transiently if at all. In fact, both primary and secondary stones can be present in the CBD for years without causing symptoms or elevating liver function enzymes [12]: This entity is described as “uncomplicated choledocholithiasis” (uCDL).
However, in approximately 40 % of cases of choledocholithiasis, persistent obstruction of either the biliary or pancreatic ducts (or both) ensues, leading to abdominal pain and tenderness, as well as both laboratory and imaging derangements. The pathognomonic manifestations of this obstruction are cholangitis and pancreatitis, respectively, and, when caused by obstructing gallstones, are termed “complicated choledocholithiasis” (cCDL). In fact, over 50 % of patients presenting with ascending cholangitis have CBD stones [24]. The relatively high incidence of symptomatology seen in choledocholithiasis, as well as the morbidity of both cholangitis and pancreatitis [25–28], have been used as arguments for routine interrogation of the CBD in cases of cholelithiasis and clearance of it when choledocholithiasis is found.
Risk factors for cCDL include older age, nonelective admission, history of alcohol abuse, male gender, obesity, and Asian/Pacific Islander race [29]. Patients with cCDL have also been shown to have a 1.5× increased odds of mortality compared to those with uCDL [29].
In general, choledocholithiasis is diagnosed by a combination of history, physical exam, and imaging modalities. Importantly, 2–3 % of patients who have had a cholecystectomy will have retained stones implying the lack of gallbladder in a patient cannot rule out the possibility of choledocholithiasis [7]. Patients with uCDL present with symptoms related to cholelithiasis in the form of either biliary colic or cholecystitis. Physical exam findings will also be driven primarily by the underlying gallbladder pathology, and may range from normal (mild biliary colic) to severe tenderness with Murphy’s sign (acute cholecystitis).
Transient obstruction of the CBD may lead to accumulation of conjugated bilirubin in the serum, with resultant jaundice and scleral icterus. Although painless jaundice and weight loss may occasionally be seen as a result of choledocholithiasis, this constellation of symptoms is much more commonly associated with pancreatobiliary malignancy [25]. A palpable gallbladder on physical exam, known as “Courvoisier’s sign”, can also rarely be seen in the setting of a stone obstructing the CBD but is more commonly associated with other obstructive processes that cause more chronically elevated intraductal pressures such as biliary malignancy [30].
Patients with suspected biliary pathology should have liver function tests (LFTs) obtained routinely, including total bilirubin (TB), direct (conjugated) bilirubin, aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP) and Gamma-glutamyl transpeptidase (GGT). Choledocholithiasis is most strongly suspected in the setting of an elevation of ALP, as well as a direct hyperbilirubinemia. In a study of 1002 patients who underwent laparoscopic cholecystectomy (LC) after having preoperative LFTs and a variety of diagnostic imaging modalities, Yang and others found that the value of liver assays is primarily in ruling out CBD stones, as they have a 95 % or greater negative predictive values individually and a combined negative predictive value of nearly 98 % [31]. A normal GGT alone was also shown to have a nearly 98 % negative predictive value but none of the individual or combined assays had greater than 28 % positive predictive value with TB being the highest at 27.4 % [31].
Other studies have found positive predictive values in the 30 % to nearly 60 % range if minimum thresholds are set for lab values above those traditionally considered abnormal [32–34]. This finding is likely due to increased elevation of liver enzymes as the time and severity of obstruction increases [24]. In the most current American Society for Gastrointestinal Endoscopy (ASGE) treatment guidelines, Maple and others note that the true negative rate (specificity) of total bilirubin has been shown to be 60 % at 1.7 mg/dL but it rises to 75 % if the cutoff is raised to 4 mg/dL [32]. However, the applicability of this threshold is limited because the mean total bilirubin level in patients with choledocholithiasis has been reported to be between 1.5 and 1.9 mg/dL, and less than one-third of patients will have a TB of 4 mg/dL or more [32–34]. Fractionating bilirubin can be helpful when a background of indirect hyperbilirubinemia is suspected (e.g., hemolytic disorders or Gilbert’s Disease) [24].
Certain subgroups of patients may not benefit from LFT determination. Specifically, one study evaluating patients undergoing elective LC found that preoperative LFTs do not alter management beyond the course that would be determined by history, physical exam, and transabdominal ultrasound (US) exam alone. This study concluded that routine LFT determination in this patient population was not cost effective [35].
Imaging is mandatory in the work up of suspected biliary disease, and affords valuable information about both the gallbladder and CBD. Because direct visualization of a CBD stone by imaging is rare, CBD dilation has been used as the primary surrogate radiographic marker for choledocholithiasis. However, both the definition and grading of pathologic CBD dilation remain debated. Many factors other than obstruction influence the CBD diameter, including age, prior cholecystectomy, and prior sphincterotomy. The ASGE guidelines suggest that a diameter of 6 mm or greater (gallbladder in situ) is a strong predictor of CBD stone obstruction. In the three studies cited by the ASGE [36–38], the weighted mean of normal CBD diameters was 3.7 mm [39]. In a younger population of 830 consecutive blood donors between 18 and 65 years old, with gallbladder in situ, the mean CBD diameter was 2.7 ± 1.2 mm (SD) and none of the study subjects had a CBD greater than 7 mm [40]. Another study found that in 30 patients over age 80, the average CBD diameter was 5 ± 1.1 mm (SD) with a range of 3.9–7.1 mm [37]. In the same study, the authors found that the CBD dilates by 0.04 mm/year [37].
Other studies have also investigated the clinical importance of the CBD diameter. In a 2011 paper, Urquhart et al. emphasize that the 6 mm diameter guideline should not be understood as an “inflection point” above which risk of choledocholithiasis absolutely increases. On the contrary, studies have shown that risk of CBD stone increases linearly with increased CBD diameter [41, 42]. In Hunt’s study of CBD diameter in 870 patients, 85 were shown to have CBD stones and almost half (42) had CBD diameter less than 6 mm. For CBD size 0–4, 4.1–6, 6.1–8, 8.1–10, and >10 mm the respective percentages of positive CBD stone finding were 3.9, 9.4, 28, 32, and 50 [41]. In another study, Boys et al. found that the rate of stones among patients presenting with acute cholecystitis and CBD diameter less than 6 mm and 6–9.9 mm was the same (14 %). At a CBD diameter above 10 mm, still only 39 % of patients had confirmed stones. They also found that US-based selection of patients for additional imaging via Magnetic resonance cholangiopancreatography (MRCP) resulted in a near 90 % negative rate; however, there was a delay in care of almost 3-days [43]. Therefore, instead of thinking of the CBD as either dilated or not dilated, it is perhaps best for clinicians to view the diameter of the CBD and risk of CBD stone on a continuous spectrum and to retain a high index of suspicion even if CBD diameter is less than 6 mm, especially in a younger patient.
The various imaging modalities employed in the diagnosis of choledocholithiasis possess unique combinations of information, invasiveness, and cost. Transabdominal US is useful for diagnosing both cholelithiasis and choledocholithiasis, and should be considered as a first imaging modality due to favorable accuracy, least invasiveness, and low cost. Although US has poor sensitivity for visualization of stones in the CBD [24, 44–48], the modality is very sensitive for detecting CBD dilation, [24, 49–52]. Furthermore, when a stone is detected in the CBD by ultrasound, the study boasts of a specificity of 100 % for the diagnosis of choledocholithiasis, [48].
Endoscopic retrograde cholangiopancreatography (ERCP) involves a more invasive and costly means by which to diagnose choledocholithiasis, but offers the benefits of additional information and potential therapy. For these reasons, ERCP is often employed as a follow-up procedure in the United States when choledocholithiasis is suspected. Imaging the biliary tree during ERCP is accomplished with retrograde cholangiography via cannulation of the ampulla of Vater. Advantages of ERCP include favorable test performance characteristics for diagnosing choledocholithiasis, (sensitivity 89–90 % and specificity is 98–100 % [53, 54]) and the ability to perform therapeutic interventions such as stone lithotripsy, extraction, and sphincterotomy. Additional diagnostic maneuvers such as CBD brushings may also be accomplished. The main disadvantages of ERCP include availability, cost, and invasiveness. ERCP is usually performed under conscious sedation, although general anesthesia is sometimes required. Concomitant therapeutic interventions may lead to important morbidities, such as bleeding, pancreatitis, and biliary or bowel perforation. Andriulli et al. published the most comprehensive review of prospective ERCP morbidity and mortality data in 2007—including 16,855 patients in 21 studies. The cumulative rate of complications was 6.85 % (CI 6.46–7.24 %), with pancreatitis being the most common event (3.47 %) and infections and bleeding being second and third (1.62 % and 1.34 % respectively). Perforations occurred infrequently at 0.6 % and deaths occurred in 0.33 % of patients. Mortalities were caused by pancreatitis, infection, bleeding, and perforation at approximately equal rates. There were additional complications related to cardiovascular events or anesthesia-related effects that brought the pooled complication rate up to 8 % [55].
The potential risk of complications related to ERCP illustrates the importance the importance of considering less invasive imaging modalities for choledocholithiasis and other biliary pathology when evaluating patients for possible ERCP intervention. When first described in the 1960s, ERCP represented an exciting, minimally invasive alternative to both imaging and instrumenting the biliary tree in the pre-laparoscopy era [56]. However, with the advent of advanced laparoscopy, and in particular laparoscopic approaches to the CBD, enthusiasm for ERCP at our center and others has waned (see treatment) [57, 58].
MRCP is an imaging technique that uses the high water content in bile to produce 2D or 3D images of the biliary tree similar to those obtained with more invasive methods such as ERCP [59]. MRCP can image the biliary tree with high precision because bile and pancreatic secretions have high water content and appear white on heavily T2-weighted images against a dark background of suppressed high-fat tissues. Importantly, MCRP images can be captured without the use of contrast or dyes [60]. In two separate meta-analyses, MRCP was found to have 85–92 % sensitivity and 93–97 % specificity for the detection of CBD stones [61, 62]. MRCP has also been shown to be equivalent to ERCP, for diagnosing biliary tree obstructions [63]. Despite these findings, MRCP is limited in detecting both smaller stones (<5 mm) and sludge. Furthermore, MRCP is challenging in individuals with a body mass index >40 kg/m2 [64–66]. Cost, time, frequent lack of availability, and inability to provide interventions all limit the utility of MRCP as a first-line imaging modality for the detection of choledocholithiasis. The greatest benefit from MRCP occur in cases of either laboratory or sonographic evidence of ductal obstruction without confirmation of choledocholithiasis or in cases in which ERCP is technically difficult or impossible (e.g., following rouy-n-y gastric bypass surgery) or unavailable.
Endoscopic ultrasound (EUS) uses specially designed echoendoscopes that take advantage of the close proximity of the duodenum and stomach to the biliary tree for imaging of the extrahepatic biliary anatomy. Three separate meta-analyses published between 2006 and 2008 reported pooled sensitivities of 89–94 % and specificities of 94–96 % for detecting CBD stones [61, 67, 68]. Although more invasive than MRCP, EUS remains safer than ERCP as a diagnostic modality. A prospective study by Canto and colleagues of 64 consecutive patients found EUS had a complication rate of 1.6 % versus 9.4 % for diagnostic ERCP [69]. The use of EUS before ERCP has been shown to significantly reduce the need for ERCP and its subsequent complications with the main drawback being the need for two procedures in the stone-positive EUS group [70]. Many endoscopists can perform ERCP if EUS is positive at same setting. EUS has also been shown to have value in finding undetected stones in patients at intermediate risk of choledocholithiasis [71]. In general, cost, availability of resources, and clinician experience will generally guide decisions on whether to employ ERCP or EUS in particular circumstances, as they have both been shown to have equivalent sensitivity and specificity [61].
Conventional and helical (spiral) CT scanning as well as CT cholangiography have been studied for the detection of choledocholithiasis. However, the utilization of radiation and contrast exposure in these studies have limited the use of these diagnostic modalities. Conventional CT scans have been found to be superior to the United States for diagnosing CBD stones, however these studies are more than 20 years old and most US hospitals now employ more advanced US imaging as well as spiral CT scanners [50, 52, 72]. In a 2000 study of 51 patients with suspected choledocholithiasis, Soto and others showed that oral contrast-enhanced CT cholangiography had 92 % sensitivity for detecting CBD stones, compared to 96 % for MR cholangiography—significantly better than the 65 % sensitivity of unenhanced helical CT [73]. The most recent study using multidetector helical CT scanning technology, published in 2013, found that unenhanced helical CT is 85 % sensitive for the detection of CBD stones with the primary limitations being radiopacity of stones and stone size less than 5 mm [74]. A 2006 study of combined unenhanced and contrast-enhanced helical CT found 71 % sensitivity in detecting CBD stones, however less than half of the cholesterol stones were detected by CT, leading the authors to conclude that CT might not be the ideal detection modality in Western countries where cholesterol stones are most common [75]. Additionally, coronal reconstruction does not improve the diagnostic efficacy of CT [76]. Given the expense, and radiation/dye exposure, the role of CT in diagnosing suspected CBD stones will likely remain limited. Clinicians should not rule out a small or radiolucent stone in a symptomatic patient where CT scans are negative.
In addition to the aforementioned nonoperative imaging techniques, both IOC and intraoperative laparoscopic ultrasound (LUS) may be performed during the course of cholecystectomy to diagnose choledocholithiasis. IOC involves the injection of iodinated contrast dye into the extrahepatic biliary tree via either the cystic duct or gallbladder for fluoroscopic imaging. Although methods for delineating the anatomy of the biliary tree were published as early as 1919, Mirizzi first described IOC in the 1930s as a way to visualize retained stones and other defects during open cholecystectomy [77, 78]. Mirizzi and other authors in the 1930s recognized that IOC was also useful for diagnosing iatrogenic bile duct injuries [78, 79].
Despite the early recognition of the value of IOC, it remained a technique routinely utilized by only a quarter of surgeons into the 1970s, due in large part to the time that it added to open cholecystectomy procedures [80, 81]. IOC is not particularly technically challenging (either open or laparoscopically), but the 20–30 min it took to setup, take, and develop the static films was a major hurdle to widespread adoption. This limitation was at least partially mitigated by the advent of mobile C-arm high-resolution image intensifier fluoroscopic units, which took total procedure time down to a mean of 16 min and significantly improved image accuracy [81–83]. Yet, a 2012 study of 177,000 cholecystectomies performed in Texas found drastically wide variation in the use of IOC, both among individual surgeons (2.4–98.4 % of cases) and hospitals (3.7–94.8 % of cases), with an overall IOC rate of 44 %[84].
There are two issues to consider when evaluating IOC in patients with suspected choledocholithiasis: (1) Does routine IOC improve the overall safety of LC? and (2) What is the utility of IOC in patients with suspected choledocholithiasis? The first question remains highly debated because the data are mixed as to whether IOC identification of biliary structures improves safety. A recent meta-analysis of eight randomized studies with 1715 patients found that there was insufficient level-one evidence to support or abandon the use of IOC. This finding resulted largely due to the studies being underpowered for detecting differences in bile duct injury rates, which occur only a fraction of one percent of the time [83]. In fact, it has been suggested that a randomized trial would have to include between 12,000 and 30,000 patients in order to be sufficiently powered to detect this difference [85, 86]. It is therefore unlikely that a definitive answer to this question will ever be found. Instead, other authors have looked at nonrandomized population-based trials. One recent review of six large nonrandomized studies found that the data is conflicting in that half of the studies showed a safety benefit, while half did not [87]. However, the largest studies suggest that routine IOC could prevent one ductal injury in every 500 operations, thereby roughly halving the risk of ductal injury during cholecystectomy [87]. This data point is particularly interesting because the rate of iatrogenic bile duct injuries in open cholecystectomies was reportedly 0.2 % and the LC duct injury rate is 0.3–0.6 % or about double that figure [88–91].
A separate issue involves the role of IOC in diagnosis and treatment of patients with suspected choledocholithiasis. The data suggests that IOC is very effective at identifying stones in the CBD as demonstrated in a recent meta-analysis, which found IOC had a pooled sensitivity of 0.87 (95 % CI 0.77–0.93) and a pooled specificity of 0.99 (95 % CI 0.98–0.99) [92]. Since the advent of endoscopic techniques for stone removal, the most commonly employed method for management of suspected choledocholithiasis has been a two-stage approach, where ERCP is performed first to find and remove CBD stones and a follow-up LC is performed for definitive treatment of the gallbladder disease [93]. In this context, the value of IOC is limited, since its use would be in detecting rare retained stones after ERCP or stones that had subsequently migrated into the CBD in the interval between ERCP and LC. However, several recent papers have shown equivalent clinical results and superior economics with a single-stage approach where LC is combined with IOC and subsequent laparoscopic common bile duct exploration (LCBDE) [94] (discussed below). In our view, the best role for IOC and LUS is in complete laparoscopic management of CBD stones.
In experienced hands, laparoscopic ultrasonography (LUS) can certainly play a similar though less invasive role in single-stage treatment of CBD stones. In LUS, an ultrasound transducer is introduced through a 12 mm port during LC, where it is used to identify biliary tree structures and stones in the CBD. Although it requires specialized laparoscopic ultrasound equipment, compared to IOC, LUS is faster, less expensive, less invasive, and avoids the risks of radiation and iodinated dye exposure [95]. For detection of CBD stones, LUS is equivalent to IOC with a recent meta-analysis showing pooled sensitivity of 0.87 (95 % CI 0.80–0.92) and a specificity of 1.00 (95 % CI 0.99–1.00) [92]. LUS has also been utilized successfully as a routine intraoperative prescreening tool for determining which patients get selective IOC [85]. Finally, LUS has demonstrated the capacity of avoiding intraoperative bile duct injuries [85]. In this case, LUS is superior to IOC in that a ductotomy (potentially of a misidentified CBD) is not necessary to delineate biliary anatomy.
In summary, abdominal US should be the first imaging modality used in all patients with suspected biliary pathology, regardless of suspicion for choledocholithiasis. Additional imaging and diagnostic modalities should be chosen based upon risk, index of suspicion for cCDL and availability of local expertise. The routine use of ERCP as a diagnostic modality is not justified due to its cost and invasiveness and because both IOC and LUS at surgery may be just as effective at identifying choledocholithiasis. The advantages and limitations of the aforementioned imaging modalities are summarized in Table 14.1.
Table 14.1
Imaging modalities for detection of CBD stones
Imaging modality | Advantages | Disadvantages | Recommended application | Refs. |
|---|---|---|---|---|
Transabdominal ultrasound | • Least invasive | • Poor sensitivity for visualizing stones in CBD | Use in all patients with suspected CBD stones | |
• Lowest cost | ||||
• Highly sensitive for CBD dilation | ||||
MRCP | • Less invasive | • Expensive | Use when labs and/or US suggest obstruction in the absence of gallstones or when ERCP is difficult or impossible | |
• No contrast | • Time consuming | |||
• Highly sensitive and specific | • Limited utility in obese patients | |||
• Limited ability to detect small stones | ||||
• Limited availability | ||||
CT scan | • Fast | • Expensive | Not recommended for use and negative CT should not rule out CBD stone | |
• Widely available | • Radiation exposure | |||
• Moderate sensitivity and specificity | • Contrast exposure | |||
• Limited ability in cholesterol stones | ||||
Endoscopic ultrasound | • Safer than ERCP as diagnostic modality | • More invasive than MRCP | Use in combination with ERCP only when ERCP is chosen first treatment modality | |
• Highly sensitive and specific | • Requires sedation and experienced endoscopist | |||
• Optional immediate intervention | • 1.6 % complication rate | |||
ERCP | • Gold standard for imaging the biliary tree | • Most invasive nonoperative approach | Imaging and intervention in acute cholangitis or gallstone pancreatitis complicated by cholangitis and/or biliary obstruction | |
• Highly sensitive and specific | • Requires sedation and experienced endoscopist | |||
• Immediate intervention | • 8 % complication rate | |||
Laparoscopic ultrasound | • Equivalent to IOC without dye or radiation | • Requires specialized equipment and training | Alternative to IOC or screening tool for IOC during complete laparoscopic management of CBD stone | |
• Faster than IOC | • Time consuming in OR | |||
• Highly sensitive and specific | ||||
Intraoperative cholangiography | • Highly sensitive and specific | • Highly invasive | Use during complete laparoscopic management of CBD stone | |
• May improve safety of LC | • Time consuming in OR | |||
• Dye exposure | ||||
• Radiation exposure |
Clinical Decision Making and Treatment
Fundamentally in cases of suspected or documented choledocholithiasis, the goals of treatment are to clear the CBD of stones if present and to remove the gallbladder. The first step in the aforementioned process involves determining the likelihood of choledocholithiasis [96, 97]. The literature is replete with predictive models for choledocholithiasis [6, 29, 31–33, 48, 98–101]. For a thorough example of such an algorithm, the reader is referred to the 2010 ASGE recommendations [24]. In these recommendations, Maple and co-authors present three predictor categories: very strong, strong, or moderate. Clinical factors such as patient demographics, physical exam findings, labs, and imaging, fit into these predictor categories (Table 14.2). Risk of CBD stone is categorized as high, medium, or low, based on the presence of various predictors (Table 14.3). Treatment is then advised based upon the risk stratification [24].
Table 14.2
Predictors of choledocholithiasis
Very strong | Strong | Moderate |
|---|---|---|
CBD stone on transabdominal US | Dilated CBD on US >6 mm with gallbladder in situ | Abnormal liver biochemical test other than bilirubin |
Clinical ascending cholangitis | Total bilirubin 1.8–4 mg/dL | Age older than 55 years |
Total bilirubin >4 mg/dL | Clinical gallstone pancreatitis |
Table 14.3
Risk of common bile duct stone
High (>50 %) | Moderate (10–50 %) | Low (<10 %) |
|---|---|---|
Presence of any very strong predictor | Presence of any combination of predictors other than those for high | No predictors present |
Presence of both strong predictors |
Despite these and other expert recommendations, there is currently no consensus approach to patients with suspected choledocholithiasis. Rather, management of these patients is based upon index of suspicion for CBD stones, availability of both resources and expertise, and local referral patterns (Fig. 14.2). Fundamentally, three strategies may be identified. The first option involves inpatient admission of patients at mild to moderate risk of choledocholithiasis (as evidenced primarily by laboratory derangements) for serial laboratory evaluation. ERCP is performed in patients with either persistent or worsening laboratory derangements. By contrast, patients in whom laboratory derangements improve are taken for LC without CBD imaging (the presumption being that the CBD stone has passed). This particular strategy is lengthy, costly, and dissatisfying to patients, who must be admitted, additional blood work obtained, and definitive surgery delayed. Furthermore, multiple studies have shown that patients classified as high likelihood of CBD stone (by ASGE recommendations) have a 40–80 % rate of actual stone on EUS or ERCP, with faster timing being postulated as the reason for better results [102, 103].
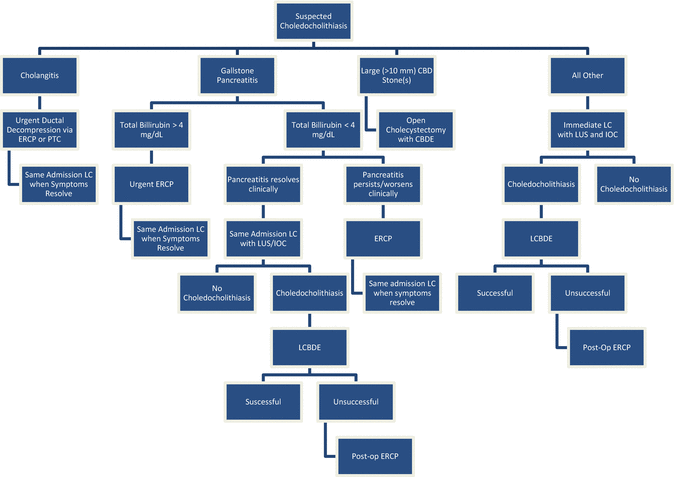

Fig. 14.2
Algorithm for management of suspected choledocholithiasis. Choledocholithiasis is suspected in the presence of cholelithiasis plus (1) jaundice, (2) direct hyperbilirubinemia or (3) dilated common bile duct diameter. ERCP endoscopic retrograde cholangiopancreatography, PTC percutaneous transhepatic cholangiostomy, LC laparoscopic cholecystectomy, LUS laparoscopic ultrasound, IOC intraoperative cholangiogram, CBDE common bile duct exploration, LCBDE laparoscopic common bile duct exploration
The second management strategy, or the “two-stage approach,” involves both routine ERCP and LC as separate procedures. In this strategy, ERCP functions to access, interrogate, and clear the CBD, including various combinations of techniques such as cholangiography, stone extraction, lithotripsy, and biliary sphincterotomy. Typically, ERCP is performed first and routinely, followed by LC. However, ERCP may also be used selectively following cholecystectomy in patients with IOC findings suggestive of choledocholithiasis. Although this latter approach carries the possibility of a third, open surgery being needed in the case of failed ERCP, this risk is exceedingly low, as contemporary technology is highly effective at clearing even larger (e.g., 8–10 mm) stones endoscopically [104–106]. US national healthcare survey data indicate that greater than 90 % of patients with CBD stones are managed using the two-stage approach. Although the order of the procedures is not clear, data suggest that ERCP first is the most popular method [107, 108].
The main advantage to the two-stage approach involves endoscopic clearance of the CBD. Prior to laparoscopic surgery, there was no advantage to pre-cholecystectomy stone removal because open bile duct clearance was common and either as good or better than ERCP at clearing CBD stones [109, 110]. However, the advent of LC rendered the CBD inaccessible by traditional means. As a result, preoperative or postoperative stone clearance with ERCP gained popularity as LC became more common.
However, as both the practice and efficacy of laparoscopic CBD clearance increased, the primary advantage of the two-stage approach has come under scrutiny. Because the two-stage approach involves at least two separate procedures, time, resources, costs, and complications are increased. The main risks of ERCP have been discussed earlier and involve duodenobiliary reflux, pancreatitis due to accidental cannulation of the pancreatic duct, duodenal perforation, and intraluminal massive hemorrhage from injury to the gastroduodenal artery.
Furthermore, as many as 65–80 % of patients with suspected choledocholithiasis will be negative for stones on ERCP/EUS, rendering the procedure unnecessary [103, 102]. Even after stones have been confirmed intraoperatively with IOC, only 50 % of post-LC ERCPs are positive for CBD stones [111], presumably because either the stone has passed in the interim or was misdiagnosed by IOC.
Finally, as many as one-third of patients managed with the two-stage, ERCP first approach, never end up having a cholecystectomy. Although biliary sphincterotomy may prevent further episodes of cCDL, both biliary colic and cholecystitis are still possible as long as the gallbladder remains in situ. The incidence of recurrent biliary symptoms is significantly higher in patients following endoscopic sphincterotomy who are discharged with gallbladder in situ versus those who receive same admission cholecystectomy [112, 113]. A prospective study of patients who had LC within 72 h of ERCP versus those who waited 6–8 weeks found that the group who waited had a 36 % rate of repeat complications compared to 2 % in the LC within 72 h group [114]. In a retrospective study at our institution, our colleagues showed that of 24 patients discharged after medical management of gallstone pancreatitis with specific instructions to return for LC, only seven (29 %) returned for definitive treatment of their gallstone disease [112].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








