ASA physical status 1
A normal healthy patient
ASA physical status 2
A patient with mild systemic disease
ASA physical status 3
A patient with severe systemic disease
ASA physical status 4
A patient with severe systemic disease that is a constant threat to life
ASA physical status 5
A moribund patient who is not expected to survive without the operation
ASA physical status 6
A declared brain-dead patient whose organs are being removed for donor purposes
The ASA classification has enjoyed its widespread use by its simplicity. Being fairly nonspecific allows it to be memorized by anesthesia personnel and be frequently utilized. However, the limitations are plentiful. One limitation of the ASA class is that it does not take into account the nature of the surgery [24], the experience of the anesthesiologist or the surgeon, and the type of facility in which the surgery is being performed [25–27]. Another limitation of the ASA system is that it does specifically not account for certain patient factors such as age, sex, body mass index, or pregnancy [25–27]. In addition, there is significant variability in interpretation of the categories among anesthesiologists [28].
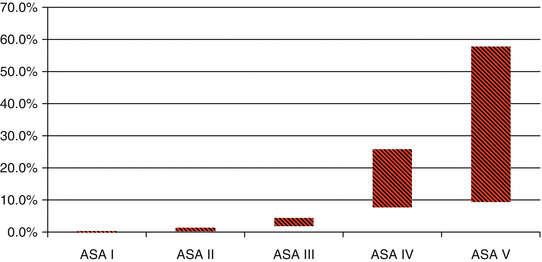
The ASA system has been studied for operations pertinent to biliary disease. In a study of the National Surgical Quality Improvement Program (NSQIP) database, the authors retrospectively analyzed data from 2005 to 2008 in 65,511 cholecystectomies performed. Of these patients, 58.2 % of surgeries were in patients classified as ASA 2, and 2.6 % were performed in patients classified as ASA 4 (Fig. 11.2) [29]. This finding may signify that patients with acute cholecystitis who are described as ASA 4 are often undergoing percutaneous cholecystectomy instead of receiving cholecystectomy.
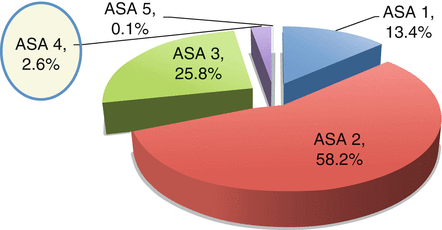

Fig. 11.2
ASA class of cholecystectomies in the National Surgical Quality Improvement Program from 2005 to 2008. Figure from Ingraham et al. [29]
The limitations of the ASA system are seen in another study of biliary patients. In A retrospective study from 2005 to 2010 showed that sixty-one “high-risk” patients were treated for acute cholecystitis. In this study, 80 % of those who were ASA 4 received percutaneous cholecystostomy, while only 4 % of those who were ASA 3 received this intervention [30]. This observation in not explained by the ASA system which does not examined the reasons for a given procedure. However, one must infer that clinicians in this study might have tended to perform less invasive procedures in patients they deemed to be sicker. The study also noted a 17.2 % mortality rate in the percutaneous group compared to a zero percent mortality in the cholecystectomy group (p = 0.02). The study additionally revealed no differences in age, APACHE II score, Physiological and Operative Severity Score for the enumeration of Mortality and Morbidity (POSSUM), or severity of cholecystitis between the percutaneous group and the cholecystectomy group.
Consequently, ASA classification has been unsuccessful in stratifying patients for possible surgery with acute cholecystitis. There are no prospective randomized studies evaluating outcome after cholecystectomy in patients who are ASA class 4. Additionally, studies have been poor in gleaning whether the ASA class or another feature influenced the need for surgical vs. nonsurgical interventions. Given the limitations of the ASA class system, this alone should not be used to determine operative risk in patients with acute cholecystitis.
Age
A patient’s age is also often used to determine operative risk for many operations, including acute cholecystitis. In a retrospective study evaluating patients with emergency operations, Matsuyama and colleagues found that age over 80 was associated with increased morbidity but not mortality [31]. In a separate retrospective study of 411 patients treated specifically for acute cholecystitis, 17 % were 80 years or older [32] and of these elderly patients, more were observed to present with gangrenous cholecystitis (44 % vs. 31 %; p = 0.033), more complications (31 % vs. 13 %; p < 0.001), and higher mortality (4 % vs. 1 %; p = 0.038). After adjusting for comorbidities, age was still found to be independently associated with poor outcomes after cholecystectomy.
In contrast, in a larger NSQIP study of 15,248 patients aged 65 years or older who underwent elective cholecystectomy, the overall mortality rate was 0.9 % [33]. Elective ambulatory cholecystectomies were associated with decreased mortality (0.2 % vs. 1.5 %, p < 0.001) and decreased complications.
Age may also influence the time to treatment and the type of treatment a patient undergoes in acute cholecystitis. In another retrospective database study of 29,818 Medicare patients admitted for acute cholecystitis, the authors observed that 25 % of patients did not undergo cholecystectomy at their first admission. In the study, the authors also noted a 38 % gallstone-related readmission rate compared to a 4 % readmission rate in those who received cholecystectomy (p < 0.0001) [6]. In this study, failure to perform cholecystectomy was associated with an increased 2-year mortality (Fig. 11.3). These data would suggest that laparoscopic cholecystectomy should be performed in earlier in the elderly if the patient can tolerate the surgery. However, since the cause of the mortality is not determined from these data, one can not definitively conclude whether the delays in treatment contributed to mortality or whether the patient had serious other life-threatening illnesses that were more responsible for true attributable mortality than the biliary disease.
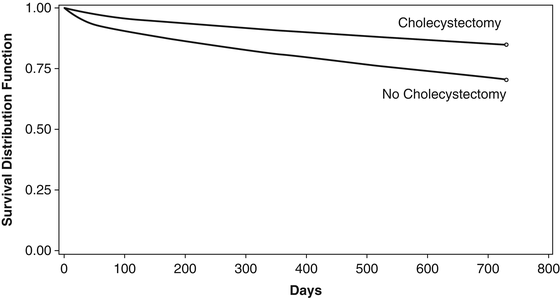

Fig. 11.3
Kaplan Meier curves for patients who received cholecystectomy at index admission and those who did not. Figure from Riall et al. [6]
Critical Illness Scores
Critically ill patients are at considerable risk for biliary disease for several reasons. These patients are predisposed to impaired tissue perfusion due to decreased cardiac output from hypovolemia or inadequate cardiac function due to coronary disease or suboptimal contractility. These factors render them susceptible gallbladder mucosal ischemia which can lead to secondary acalculous cholecystitis or biliary stasis and acute calculous cholecystitis [34, 35].
Illness severity scores can sometimes capture a patient’s risk for mortality in the ICU setting but does not specify how much risk a particular operation can confer. A retrospective review of critically ill patients with a mean APACHE II score of 25 possessing acalculous cholecystitis underwent open cholecystectomies [36]. These patients displayed a mortality rate of 44 % as 64 % of these patients had multiorgan failure on the day of cholecystectomy (Fig. 11.4). While the APACHE II (Acute Physiology and Chronic Health Evaluation) and the SAPS II (Simplified Acute Physiology Score) scores were not significantly different between survivors and non-survivors, the SOFA (Sequential Organ Failure Assessment) score was (9.5 vs. 12.9, p = 0.007).
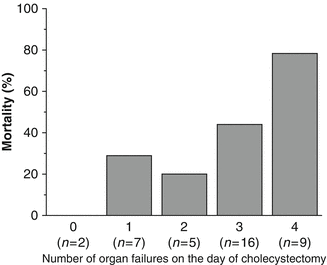

Fig. 11.4
Numbers of patients with organ failure on the day of cholecystectomy. Figure from Laurila et al. [36]
While the intervention in this study is not controlled, and all the cases were done open, the high mortality rate associated with cholecystectomy in this population of patients with acalculous cholecystitis suggests that an alternate strategy is preferred. This alternate strategy would appear to be percutaneous cholecystostomy (PC) with delayed cholecystectomy in patients with this degree of critical illness. While a cutoff of a particular APACHE score deeming a patient too high a risk for surgery is not available from these data, many clinicians now feel that PC to be the preferred treatment for acalculous and perhaps even calculous cholecystitis in critically ill patients who secondarily develop the biliary condition. A review of 55 patients treated in this fashion demonstrated a mortality rate of 5.4 %, a number far more favorable than the mortality in the study by Laurila et al. [37]. Notably, in the study by Spira et al., 95.7 % of patients had resolution of their symptoms.
Another study of 42 patients by Melloul et al. with sepsis and either calculous or acalculous cholecystitis compared emergent cholecystectomy to PC [38]. The mortality rates were not significantly different between the PC and emergent cholecystectomy groups (13 % vs. 16 %, p = 1). However, PC was associated with significantly less complications (8.7 % vs. 47 %, p = 0.011). Two patients who did not respond to PC required emergent cholecystectomy secondary to gangrenous cholecystitis. Incidentally, the likelihood of gangrenous cholecystitis is reported to be higher (40–80 %) in patients with acalculous cholecystitis compared to calculous cholecystitis (2–31 %) [36, 39–43]. In aggregate, these data strongly suggest that PC in this critically ill patient population with delayed cholecystectomy may be the safest option.
Cardiovascular Disease
Specific for emergency procedures vary greatly based on the type of cardiovascular illness and type of procedure. In the study by Matsuyama et al. examining outcomes patients after emergency surgery, cardiovascular surgery, ischemic heart disease, shock state, deteriorated consciousness, Chronic Obstructive Pulmonary Disease (COPD), hemorrhage requiring transfusion, surgeries at night, and surgeries over 2 h contributed to postoperative mortality and morbidity [31]. In particular, the highest risk factors for mortality of these comorbidities were shock, deteriorated consciousness, COPD, and ischemic heart disease in that order. Notably, ischemic heart disease conferred a 3.8 times, as measured by odds ratio, increased risk for mortality.
Importantly, clinicians may approach patients differently if they possess certain comorbidities. In the NSQIP study by Ingraham et al. of 65,511 cholecystectomies performed between 2005 and 2008, the authors observed that 7.7 % of the patients had CAD and 0.71 % of patients had congestive heart failure. In both these groups, the authors observed that the groups with the comorbidity were more likely to undergo open cholecystectomy than laparoscopic cholecystectomy [29]. The reasons for this discrepancy are not clear from the data. Speculated reasons include whether the patients with the comorbidities presented later with the acute cholecystitis or the clinicians were biased away from laparoscopic surgery in this population. As data do not explain this effect, possible reasons, which are again speculative, could show this result may be due to a perception that the patients may not tolerate the laparoscopic surgery from insufflation effects or a potential prolongation of surgical duration time.
However, other studies have suggested that biliary surgery may be safe in patients with severe CAD. In a retrospective study of patients who received laparoscopic cholecystectomy, the authors compared those with severe cardiovascular disease (New York Heart Class III and IV) to those without severe heart disease and found that no significant difference existed in mortality rates between the two groups [44]. The patients with severe cardiovascular disease did experience increased hospital stay, but there was no significant difference in the morbidity rate (7.6 % vs. 4.3 %, p = 0.188).
Even patients with extreme CAD have been shown to be potential candidates for biliary surgery. Different groups have reported successful laparoscopic cholecystectomies in patients with severe heart disease including left ventricular assist devices [45–48]. A separate review of 15 patients who underwent placement of an intra-aortic balloon pump in order to undergo cholecystectomy documented a mortality rate of only 13 % [49]. A study of cholecystectomy in patients who received heart transplants showed that 72.2 % were operated on for acute cholecystitis and the overall mortality rate was only 2.2 %. Notably, in this study the mortality rate was higher for open cholecystectomy compared to laparoscopic cholecystectomy (3.6 % vs. 0.9 %, p = 0.009) [50]. The mortality rate was also significantly higher in emergent cases compared to elective cases (3.6 % vs. 0 %, p = 0.04).
Overall, the studies demonstrate that severe CAD is not an absolute contraindication to elective laparoscopic cholecystectomy but perioperative mortality rates are higher than in healthier populations. Given the high mortality rate associated with emergent operations in this group of patients, cholecystectomy in the elective setting should be considered in patients with even severe CAD if the management of their heart disease can be optimized.
Chronic Obstructive Pulmonary Disease
Severe lung disease can render a patient high-risk for elective surgery preoperatively, intraoperatively, and postoperatively. Preoperatively, severe pulmonary disease can lead to chronic deconditioning, infectious complications that necessitate antibiotic use and complicate further antibiotic regimens, and delays in presentation for non-pulmonary diseases including acute cholecystitis. Insufflation during laparoscopic surgery is associated with adverse effects on pulmonary function. These effects can be more pronounced in patients with COPD and lead to potentially aborted surgical procedures. Postoperatively, abdominal incisional pain can limit pulmonary excursion, especially if the incision is a subcostal incision as used in open cholecystectomies. Data would support that the physiologic shortcomings inherent with COPD adversely affects outcomes. In the retrospective review by Matsuyama et al., the authors observed that patients with COPD who underwent emergency surgery had an increased mortality rate of 12 % [31].
From the NSQIP database, 2.6 % of cholecystectomies done between 2005 and 2008 were done in patients with COPD [29]. A case–control study was performed to evaluate outcomes after laparoscopic cholecystectomy in patients with and without COPD [51]. The study found no differences in mortality, lengths of surgery, or lengths of hospital stay in either of the two groups. The only difference noted in the groups was that end-tidal CO2 was higher in the COPD group, despite using limited insufflation pressures of 12 mmHg. These data would suggest that in patients with COPD, laparoscopic cholecystectomy in an elective setting should be given consideration. However, this study had several limitations. Complete preoperatively pulmonary function tests were not reported for the two groups which were largely deemed to have only “mild” COPD. Activity level of the two groups was additionally not reported. Also, the groups were small and did not include any patients who needed conversion to open surgery. Nonetheless, the study does show that laparoscopic cholecystectomy can be safely performed in selected patients with COPD.
Cirrhosis
The prevalence of gallstones in cirrhotic patients is three times higher than in non-cirrhotics [52, 53]. This observation is multifactorial and has been attributed to multiple factors including hemolysis secondary to concurrent hypersplenism, decreased biliary acidity, impaired gallbladder emptying, related to high estrogen and progesterone levels, and metabolic liver failure [54].
However, the influence of cirrhosis on biliary anatomy is not always predictable. In early cirrhosis, the cirrhosis may pose no anatomical distinction for patients undergoing cholecystectomy. In more advanced cirrhosis, extreme right upper quadrant bleeding may occur during perihilar gallbladder dissection due to collateralization, scarring of the gallbladder to the liver parenchyma, coagulopathy, or ectopic vasculature. In other cases, the gallbladder may be contracted and relatively avascular. Importantly, the consistency of the liver may vary as well in terms of vascularity in these patients independent of the individual ability of the patient to create clotting factors.
Due to some of these inconsistencies, clinicians for some years have debated whether cholecystectomy should be performed laparoscopically or open in cirrhotic patients. A meta-analysis of 3 randomized controlled trials comparing laparoscopic and open cholecystectomy in patients with cirrhosis revealed decreased complications and shorter length of stay in the laparoscopic group [54]. In this study, there was no significant difference in Child-Turcotte-Pugh class (CTP) between laparoscopic and open groups. The rate of postoperative hepatic insufficiency was higher in the open cholecystectomy group, but this was not statistically significant (18.1 % vs. 7.7 %). Unfortunately, this study evaluated patients mainly to the Child class A and B whereas the differences in technical feasibility may be most pronounced in patients with advanced cirrhosis.
Other investigators have tried to examine risks in cirrhotic patients undergoing biliary surgery. A retrospective single-center review by Quillin et al. of 94 cirrhotic patients who underwent laparoscopic cholecystectomy from 2000 to 2009 showed that laparoscopic cholecystectomy could be performed safely in selected cirrhotic patients with a conversion rate of 11 %. The reasons for conversion were not enumerated in the study but appeared to be related to increased blood loss in the patients receiving open surgery. The study identified factors associated with increased morbidity to be low serum albumin, elevated INR, CTP, and number of red blood cell transfusions [55]. In this study, the mortality rate was 4 % but notably both patients who were CTP class C perished. The Model for End Stage Liver Disease (MELD) score was not associated with mortality, but was associated with morbidity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree