KEY POINTS
Survivors of critical illness experience important functional decrements and decreased health-related quality of life due to ICU-acquired weakness and a spectrum of other physical disabilities, neurocognitive and neuropsychological dysfunction.
These morbidities may not be wholly reversible and the decrement in function may be more marked in older patients, those with a greater burden of comorbid illness or longer ICU length of stay.
Poor neurocognitive outcomes have been linked to delirium, hypoxia and sedative-hypnotic use, hypoglycemia, and possibly conservative fluid management; dysfunction is similar to that of moderate traumatic brain injury and mild dementia.
Approximately one-third to one-half of survivors of critical illness will develop long-term neurocognitive impairments.
Early mobility during critical illness is safe and feasible.
ICU multidisciplinary early mobility rehabilitation programs designed for patients who had good premorbid functional status improve functional outcome at ICU and hospital discharge. The role for these programs in less functional patients at ICU admission is unclear as is the lasting effect of this early rehabilitation intervention on longer-term outcomes.
ICU self-help manual has been shown to improve physical outcomes after critical illness.
ICU diaries have been shown to improve psychological outcomes in patients after critical illness.
Neurocognitive rehabilitation has shown some early benefit on outcome and requires further study.
Family caregivers also experience psychological morbidity and are important modifiers of patient outcome over time.
ABSTRACT
An episode of critical illness is transformative. Patients suffer important new nerve, brain, and muscle injury that results in important functional limitations that affect health-related quality-of-life (HRQoL) outcomes. The spectrum of morbidity varies according to individual risks but prevalent disabilities transcend diagnostic groupings. Each patient who enters the intensive care unit (ICU) will begin to degrade his or her muscles through upregulation of different proteolytic pathways, and although the inciting stimulus, or its magnitude, may differ somewhat across patients, the result is the same. This argues for an approach to rehabilitation that is etiologically neutral and based on an understanding of molecular pathophysiology that can be mapped to functional outcome and tailored to individual need. Neuropsychological dysfunction is important and also potentially irreversible and similar to that of moderate traumatic brain injury and mild dementia. Cognitive interventions may need to follow a similar rehabilitation model to those proposed for ICU-acquired weakness (ICUAW). Family caregivers should be part of the rehabilitation intervention as they represent important risk modifiers of short- and longer-term outcomes.
KEYWORDS
BACKGROUND
Surviving critical illness is only the beginning. Only recently has it become clear that an episode of critical illness results in long-term physical and neuropsychological dysfunction, ongoing health care utilization and incurred costs, and the risk of financial and mental health devastation of families.1-9 This acquired disability may be irreversible.2 The legacy of muscle, nerve, and brain dysfunction may necessitate a change in disposition where those who were previously living independently may require assisted living situations or comprehensive care after their critical illness.4,5 Acquired morbidity comes at significant additional cost with some reports that health care utilization after critical illness is similar to that for patients with chronic disease.2,10,11
In this chapter, we will review the important recent advances in our understanding of outcomes after critical illness and focus on newer data on functional and neuropsychological disability in patients and family caregivers and early models of rehabilitation and intervention after critical illness. Most literature remains focused on long-term outcomes after acute lung injury, but emerging data on sequelae of chronic critical illness will be included here as it adds depth to our current understanding of the spectrum of post-ICU disability. Finally, the chapter will conclude with a commentary about the future direction of outcomes work and potential rehabilitation strategies for patients and families after critical illness.
LONG-TERM OUTCOME MEASURES IN CRITICAL ILLNESS
The nature of the ICU outcomes literature has progressed from physiologic measures, mostly comprised of cardiopulmonary function, to the study of generic health-related quality of life (HRQoL). These early data suggested important decrements in physical function without a clear understanding of specific contributing factors.12 This was followed by more recent data suggesting that physical HRQoL was heavily influenced by ICU-acquired muscle wasting and weakness.13 Additional findings have been added to these observations including prevalent neurocognitive disability14 and mood disorders.15 Contemporary outcomes work focuses on functional independence5 to inform HRQoL outcomes and includes diverse patient samples comprised of different clinical phenotypes with varied and distinct outcome patterns contributing to a more comprehensive understanding of the spectrum of disability after critical illness. The spectrum ranges from the young and previously healthy,2 older with comorbid illness,4,5 elderly with preexisting functional disability,16 and the very long-term ventilated4 patient, and how these phenotypic groupings migrate with different functional dependences, mood disorders, health care utilization and disposition.
Patients with acute lung injury and acute respiratory distress syndrome (ALI/ARDS) have served as the archetype of complex critical illness and its outcomes. ALI/ARDS is a clinical syndrome of rapid onset bilateral pulmonary infiltrates and hypoxemia of noncardiac origin.17,18 In 2005, it was estimated that ALI/ARDS affected 190,600 people per year in the United States and was associated with 74,500 deaths, and 3.6 million hospital days.19 In the United States, over 100,000 patients will survive ALI/ARDS each year19; they have been the most rigorously studied group of ICU survivors to date and their outcomes will form the basis of the discussion to follow. There is an important emerging literature on longer-term outcomes in the chronically critically ill, the elderly, and sepsis patient populations, and these data will be included where relevant.
HEALTH-RELATED QUALITY OF LIFE
HRQoL is an important patient-centered outcome. However, it is intensely personal and reflects personal values. As such, it may not represent the best outcome measure to inform details of functional or neuropsychological disability and how to construct individually tailored rehabilitation programs to meet specific needs.
HRQoL is defined as a set of causally linked dimensions of health, including biologic/physiologic, mental, physical, social function, neurocognitive, and health perception.20 Measures of HRQoL assess how disease and its treatment are related to physical, social, emotional, and neurocognitive functioning and it has emerged as an important patient-centered metric of recovery from critical illness. There is emerging evidence that the degree of disability acquired after critical illness and resultant HRQoL may be variable and related to differences in premorbid functional status, burden of comorbid illness, and nature and duration of critical illness. This heterogeneity is important to consider when attempting to risk stratify patients for early mobility and post-ICU rehabilitation interventions.
Although there is some heterogeneity across different study samples of ARDS patients, there appears to be less variability in reported HRQoL in this group compared with general populations of critically ill patients.21 The following is a brief, historical overview of the emergence of the ARDS HRQoL outcomes literature that served as the sole model for outcomes after critical illness until more recently. In 1994, McHugh and her colleagues prospectively evaluated pulmonary function and quality of life to assess the relationship between pulmonary dysfunction and functional disability.22 These authors found that the Sickness Impact Profile (generic quality-of-life measure of the subject’s self-perceived physical and psychological condition) scores were very low at extubation, rose substantially in the first 3 months and then exhibited only slight improvement to 1 year. When quality of life was assessed using a lung-related Sickness Impact Profile score, only a modest proportion of the patients’ overall disability was attributed to pulmonary dysfunction. Weinert and coworkers20 also identified functional impairment in their lung injury survivors and captured disability through the Medical Outcomes Study 36-item short-form health survey (SF-36), which yields scores in eight domains including physical and social functioning, role limitations because of emotional or physical problems, mental health, vitality, bodily pain, and general health perceptions.23 While all domains of the SF-36 were substantially reduced in their study sample, the largest decrements occurred in role-physical and physical functioning and were largely attributed to global and generalized disability. Schelling et al24 made similar observations about impaired physical functioning and inferred that disability was due to pulmonary dysfunction; however, they did not assess this directly in their study. Davidson and colleagues25 assessed differences in HRQoL in ARDS survivors and comparably ill controls using the SF-36 and a pulmonary disease–specific measure (St George’s Respiratory Questionnaire [SGRQ]), to determine the degree to which perceived physical disability in ARDS survivors was related to pulmonary dysfunction. Similar to previous reports, all domains of the SF-36 were reduced and the largest decrement was in the role-physical domain. ARDS survivors had significantly worse scores on the SGRQ compared to critically ill controls, suggesting an ARDS-specific degree of physical disability but it was not clear whether this was solely related to pulmonary dysfunction or whether there were other important extrapulmonary contributors.
Angus and colleagues12 used the quality of well-being score (QWB) in a prospective cohort of ARDS survivors to measure quality-adjusted survival in the first year after hospital discharge. The mean QWB scores for their ARDS cohort at 6 and 12 months were significantly lower than a control population of patients with cystic fibrosis. When QWB was disaggregated into its component subscores, the symptom component scores of the QWB accounted for 70% of the decrement in perfect health at 6 and 12 months and the most common complaints were musculoskeletal and constitutional. In their prospective cohort study of 78 ARDS survivors, Orme and colleagues26 evaluated HRQoL and pulmonary function outcomes in patients treated with higher tidal volume versus lower tidal volume ventilation strategies. Both groups (higher and lower tidal volumes) reported decreased HRQoL in physical functioning, physical ability to maintain their roles (role-physical), bodily pain, general health, and vitality (energy) on the SF-36. The minor pulmonary function abnormalities correlated with decreased HRQoL for domains reflecting physical function.
LONG-TERM FUNCTIONAL DISABILITY
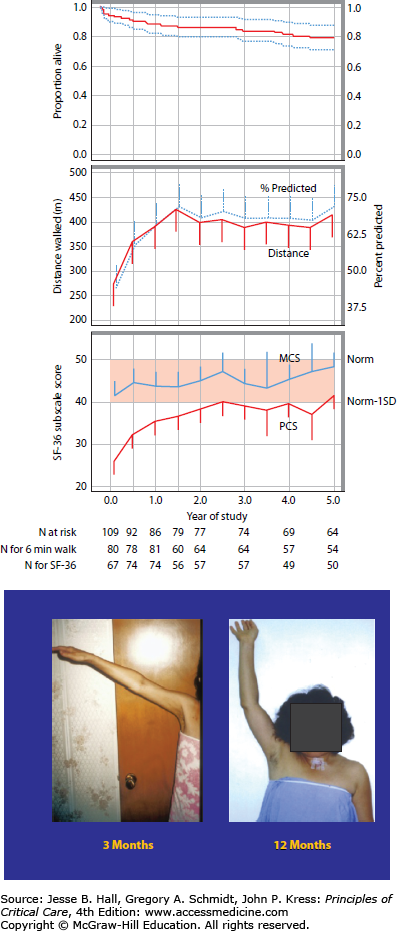
The observation of impaired physical functioning after critical illness is robust across studies and investigators and persists for long periods of time following ICU or hospital discharge and in some cases, may be irreversible. The Davidson paper25 discussed above reported outcomes at 23 months after discharge and Herridge and colleagues have documented persistent physical dysfunction at 2 and again at 5 years after ICU discharge2,27 This recent 5-year ARDS outcomes paper demonstrates that relatively young (median age 45), previously working patients with few comorbidities may not regain their precritical illness functional status nor premorbid HRQoL 5 years after ICU discharge. Physical disability, including ICUAW, and decrements in neuropsychological performance may contribute to this persistent dysfunction captured as a reduction in the physical component score (PCS) of the SF-36. This was reported as one standard deviation below an age- and sex-matched control population at 5 years after ICU discharge (Fig. 15-1A). These morbidities generated additional health care costs that were higher than predicted for this age group and more comparable to individuals with chronic disease.
FIGURE 15-1.
A. Survival, 6-minute walk distance and quality of life to 5 years after ICU discharge. Exact survival times were used for these analyses whereas deaths indicated in the consort diagram were included between scheduled follow-up visits. Top Panel: Kaplan-Meier curve to 5 years. Dashed lines represent the 95% confidence interval. Middle Panel: Distance walked in 6 minutes (meters and % predicted); distance in meters is a solid line and % predicted is a dashed line. Bottom Panel: SF-36 Subscale scores for Physical Component Score (PCS) and Mental Component Score (MCS). (Reproduced with permission from Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. April 7, 2011;364(14):1293-1304.) B. Improvement but incomplete resolution of muscle weakness at 1 year after ICU discharge.
As discussed above, quality of life may improve over months to years after ICU discharge but, on average, does not appear to return to premorbid baseline based on long-term data in ARDS patients. A recent meta-analysis of HRQoL studies in ARDS patients found lower quality-of-life scores for ARDS survivors consistent with what has been reported previously.21 In addition, HRQoL recovery in ARDS survivors was uneven across domains and time, similar to the finding of Hopkins et al.28 Despite early improvement in the mental health domains, quality of life in ARDS survivors remains significantly lower than healthy populations years after ICU discharge.21 Another meta-analysis of quality-of-life studies in general critically ill patients consistently reported lower scores than matched, normative controls at all time points (from hospital discharge to 66 months later) after ICU discharge.29 Further, they found larger decrements in the four physical domains (physical functioning, role-physical, bodily pain, and general health perceptions) compared to the mental domains (vitality, social functioning, role-emotional, and mental health). The greatest gains occur in physical functioning, social functioning, and role-physical in the first 6 months, with only modest additional improvements thereafter.29 Recent 5-year ARDS data show that there is little change in mental domains over years, but there continues to be some improvement in physical domains over time; however, these never achieve normal predicted values. The most persistently affected domains over time are those of general health and vitality and these do not improve between 1 and 5 years after ICU discharge.2
Iwashyna and colleagues found persistent reduction in functional status after sepsis and critical illness. In their older patient study sample (median age 77), they observed a high rate of new functional limitations in those who had no limits prior to their episode of sepsis (mean 1.57 new limitations; 95% CI 0.99-2.15). In those with reductions in activities of daily living prior to sepsis, they noted an important further decrement in function. They reported that neurocognitive and physical decline persisted for at least 8 years after the episode of sepsis and this represented an important and pivotal decline in the patients’ ability to live independently.5
The robust theme of acquired and persistent morbidity after critical illness was also noted in a publication by Unroe and colleagues.4 These authors evaluated outcomes, care trajectories, and health care utilization in a study sample (n = 126) requiring prolonged mechanical ventilation (median age 55). Most patients had two comorbid illnesses at the time of hospitalization and the majority were not employed or were retired or disabled. At 1 year, only 11 patients (9% of the cohort) were alive and functionally independent. Risk factors for poor outcome included the following: older age, greater burden of comorbid illness, and discharge disposition to a postacute care facility. The total cost for this cohort of 126 patients was $38.1 million. The mean cost per patient was $306,135 (SD, $285,467) for an estimated $3.5 million per independently functioning survivor at 1 year.4
HRQoL AND NEUROPSYCHOLOGICAL MORBIDITIES
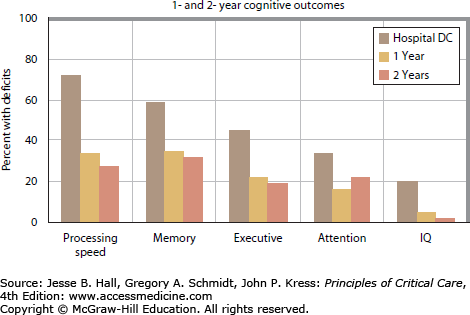
The landmark paper by Hopkins and colleagues first described neurocognitive dysfunction in ARDS survivors and its important impact on HRQoL.9 Fifty-five consecutive ARDS survivors had decreased HRQoL related to neurocognitive disability and this was noted to persist to 2 years after hospital discharge.28 These observations were confirmed by Rothenhausler and colleagues who reported that ARDS survivors with neurocognitive sequelae had worse quality of life than individuals without neuropsychological dysfunction.30 Decreased HRQoL has also been associated with psychiatric morbidities such as posttraumatic stress disorder (PTSD), which may represent yet another important contributor to subsequent disability and loss of employment.15,31,32 A more fulsome discussion of neuropsychological morbidities will follow later in this chapter.
There is clear evidence that HRQoL in ARDS survivors is adversely influenced by physical and neuropsychological morbidities. These observations have helped to elevate awareness about the important consequences of critical illness in the critical care community, but an important limitation that remains is the lack of generalizability of the ARDS outcomes literature to all ICU survivors. Rapidly accruing outcomes data from international cohorts, evaluating both functional and neurocognitive long-term outcomes, has helped us begin to understand the heterogeneous nature of reported morbidity and the complexity of interaction among physical, emotional, and neurocognitive domains in individual patients ().
NEUROMUSCULAR DYSFUNCTION
Recent research work has highlighted the concept of a continuum of weakness that begins with muscle injury documented within hours of mechanical ventilation,33 is evident with bedside testing using clinical strength measures (MRC scoring system) within 1 week of ICU admission,34 and may persist with incomplete recovery for years after ICU discharge (Fig. 15-1B).2 Muscle weakness and impaired function constitute an important morbidity of severe critical illness.
ICUAW appears to be ubiquitous in severe lung injury and also with other complex critical illnesses. Regardless of disease process, muscles and nerves are injured and this manifests as prolonged mechanical ventilation and poor functional outcomes. However, ICUAW does not completely explain functional impairment since this is influenced by many factors. In 2009, a Round Table Conference was held in Brussels, which produced a series of publications that serve as a good review on this topic including a framework for classification of ICUAW.13 An overview of the findings was included in a summary by Griffiths and Hall.35 ICUAW comprised a nerve or muscle lesion or a combination of each as outlined below and recently reviewed in detail by Latronico and Bolton.36
CRITICAL ILLNESS POLYNEUROPATHY
Bolton and colleagues first described critical illness polyneuropathy (CIP) in 198437 and reported on five critically ill patients who were having difficulties with liberation from mechanical ventilation. On electrophysiological testing, these patients had a primary axonopathy, which manifested clinically as a mixed sensorimotor neuropathy. Since this initial publication, it has become clear that CIP is very common in patients with the systemic inflammatory response syndrome (SIRS) and sepsis, with an occurrence of 70% to 100% of longer stay ICU patients. It affects the limb and respiratory muscles and facial muscles are typically spared. Limb involvement is symmetrical, most prominent in the proximal muscle groups and in the lower extremities. Detection of the true incidence of CIP is complicated by lack of consensus on surveillance, timing and nature of testing, limitations to testing because of patient sedation or poor cooperation, formal definition, and diagnostic criteria. When patients were evaluated strictly on clinical grounds for weakness, studies have reported an incidence of 25% to 36%.34,38 A systematic review on 1421 critically ill patients, reported an incidence of ICUAW of 46% (95% confidence interval 43%-49%).39 They defined patients as having ICUAW if they were evaluated using diagnostic tests (nerve conduction velocities, needle electromyography, direct muscle stimulation, histopathology of muscle or nerve tissue) or a combination of these test findings and clinical findings of muscle weakness, decreased or absent deep tendon reflexes, and/or failure to liberate from mechanical ventilation. Weakness may initially be absent or difficult to detect clinically in these patients, but subsequent electromyography (EMG) testing will demonstrate abnormalities showing an initial primary axonal degeneration of the motor neurons, followed by the sensory neural fibers, and this coincides with acute and chronic changes of denervation noted on muscle biopsies in affected patients.40
SIRS and Sepsis: CIP occurs in the context of SIRS and sepsis as shown by multiple prospective and retrospective cohort studies.41 In sepsis, the pathogenesis of CIP is linked to a perturbation in the microcirculation with resultant axonal injury and degeneration. A recent report describes increased expression of E-selectin on the endoneurial and epineurial vessels of peripheral nerves in septic patients and this has been shown to be mediated by proinflammatory cytokines such as TNF-α and IL-1.42 There is also evidence for a disruption of nerve action potential, which may be functional—and potentially entirely reversible early on—and not necessarily structural over the course of the disease.43
Hyperglycemia: ICUAW is consistently associated with hyperglycemia in critically ill surgical and medical populations.44-46 In their initial landmark publication, Van den Berghe and colleagues demonstrated that tight glycemic control reduced CIP, as defined by neurophysiologic testing, from 51.9% in control subjects to 28.7% among insulin-treated patients.44 Similar findings were noted in a predominantly medical population in a subsequent study by the same group of investigators.45 The pathophysiologic link between glucose control and neuroprotection remains unclear, although there are some emerging data that may provide some new insights. Vanhorebeek and colleagues47 found that hyperglycemia causes mitochondrial dysfunction and ultrastructural damage in the hepatocytes of critically ill patients. One might speculate that there is a similar effect on the peripheral nervous system and that protection of intact neuronal mitochondria may avert the deleterious effects of oxidant injury and apoptosis that have also been implicated in the pathophysiology of CIP.48 There may also be an important contribution from derangement of nitric oxide production. Asymmetric dimethylarginine inhibits nitric oxide production and is an independent predictor of mortality in critically ill patients. Siroen and colleagues49 showed recently that insulin modulates levels of asymmetric dimethylarginine and this may be an additional pathway by which insulin improves this outcome. Some evidence indicates that insulin inhibits proinflammatory transcription factors and may actively promote neuroregeneration during critical illness.50,51
Pharmacologic Agents: Early reports suggested a link between neuromuscular dysfunction and the use of neuromuscular blockers and systemic corticosteroids.52-54 This risk was highlighted by the observation of neuromuscular dysfunction in patients with status asthmaticus who received treatment with both agents.55 However, these relationships have not been borne out in a recent, exhaustive systematic review.56 Reports link aminoglycoside, vasopressor, and renal replacement therapy use with neuromuscular dysfunction, but since most patients have sepsis or SIRS and will receive these therapies, it is very difficult to determine any true causality.57,58 Furthermore, in a recent randomized controlled trial on the early use of paralytic therapy in patients with severe ARDS, the exposure to 48 hours of continuous paralysis did not appear to confer additional risk for weakness on the ICU survivors as assessed by the Medical Research Council score assessing strength.59
CRITICAL ILLNESS MYOPATHY
Critical illness myopathy (CIM) encompasses a variety of descriptive terms that include critical illness myopathy, acute quadriplegic myopathy, thick filament myopathy, and necrotizing myopathy. Reported incidence has varied between 48% and 96% in prospective studies that have included muscle biopsy as part of their diagnostic evaluation.58 CIM is characterized pathologically by a nonnecrotizing myopathy that is diffuse and associated with fatty degeneration of muscle fibers, fiber atrophy, and fibrosis.60 This has been described in patients with sepsis but also in those treated with corticosteroids and neuromuscular blockers. Patients will be weak, paretic, and have difficulties in weaning and may be indistinguishable from patients with CIP. Muscle biopsy allows differentiation between these lesions.
Thick filament myopathy shows a selective loss of myosin filaments in the context of significant corticosteroid or neuromuscular blocker exposure and immobility.61 Some have speculated that this may represent a precursor to acute necrotizing myopathy since this form of CIM may show progression to myonecrosis. Acute necrotizing myopathy is distinguished by extensive myonecrosis with vacuolization and phagocytosis of muscle fibers and has been most often linked to corticosteroid and neuromuscular blocker exposure and occurs in the context of multiple failed organ systems.62
The pathophysiology of CIM entails catabolism, inflammation, and derangement of membrane excitability. Protein catabolism and an increase in urinary nitrogen loss are observed in CIM. Muscle biopsies in affected patients show low glutamine, protein, and DNA levels. There is evidence for the upregulation of the calpain and ubiquitin proteolytic pathways and this occurs in concert with an increase in apoptosis.63
Inactivity in critically ill patients is linked to propagation of inflammatory mediators, which result in direct stimulation of protein loss in differentiated muscle cells, activation of a cascade of signaling events that promote oxidative injury, and disruption of insulin receptor signaling in muscle resulting in a reduction in substrate availability and impairment of myofibril growth and repair.64 IL-1, IL-6, and TNF-α have proinflammatory properties and have all been implicated in muscle degradation in critical illness and augment proteolysis and result in loss of muscle mass with a resultant decrease in muscle strength. The presence of IL-10 inhibits proinflammatory mediators and may have a role in mediating apoptosis and myocyte proteolysis.65 Some studies suggest evidence of muscle membrane inexcitability, which may be related to inactivation of sodium channels at the resting potential (sodium channelopathy). A recent report by Allen and colleagues found altered muscle-fiber excitability and evidence for muscle membrane dysfunction as the principal underlying abnormality in CIM.66
CLINICAL PHENOTYPES IN CRITICAL ILLNESS AND THE SPECTRUM OF DISABILITY
There are emerging data from recent cohort studies and administrative datasets that the heterogeneity in disability after critical illness may be organized into discrete etiologically neutral clinical phenotypes with different risks and recovery trajectories over weeks and months after critical illness. These different clinical groups, when viewed together, may comprise the spectrum of disability and facilitate the development of rehabilitation interventions by understanding how common patient traits may be used to risk stratify and to inform the needs of that specific group.
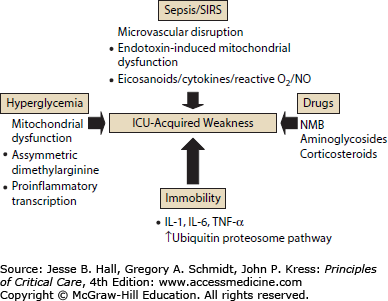
The imbalance between protein synthesis and protein degradation appears to be universal in critically ill patients. Proteolysis in diaphragm and muscles of the axial skeleton appears to be an early and ubiquitous finding.33 In their landmark work, Levine and colleagues noted that patients from very diverse clinical groupings (stroke, motor vehicle accident, drug overdose, gunshot wound) had similar muscle injury attributed to increased activity of the ubiquitin-proteosome pathway. Follow-up observations from these same investigators showed marked decreases in myosin heavy chains and atrophic AKT-FOXO signaling play important roles in eliciting the myofiber atrophy and decreases in diaphragm force generation associated with prolonged human diaphragm disuse.67 Other recent work cites induction of autophagy68 and mitochondrial dysfunction in human muscle69—observations not linked to a specific disease etiology. A recent comprehensive review on the molecular mechanisms of muscle and nerve injury in critical illness has outlined these mechanisms in more detail (Fig. 15-2).70 These important observations were not linked to a specific inciting disease and support the hypothesis that muscle injury is not specifically linked to underlying disease or etiology.
FIGURE 15-2.
Cognitive outcomes after ARDS. (Data from Hopkins RO, Weaver LK, Pope D et al: Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome, Am J Respir Crit Care Med. 1999 Jul;160(1):50-56 and Weaver LK, Collingridge D: Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005 Feb 15;171(4):340-347.)
DIFFERENTIAL REPAIR
Muscle injury may be inevitable but repair across patient groupings appears to be variable. Most muscle repair and functional recovery occurs early and stabilizes by 6 months to 1 year after critical illness.2 This variability in outcome supports the notion of a spectrum of disability related to age, comorbid disease, and ICU length of stay. Current evidence supports these are key determinants of functional outcome and compromised HRQoL.4,5,71-75 Patient demographic and clinical characteristics may serve as proxy measures for nerve and muscle reserve and/or comorbid organ dysfunction that existed prior to the episode of critical illness.
SARCOPENIA OF AGING
Sarcopenia is defined as a decline in skeletal muscle mass, strength, power, and physical functioning in association with aging.76 Sarcopenia contributes significantly to physical inactivity, functional disability, increased health care utilization, costs, and mortality in older patients.77 The muscle wasting and weakness observed in survivors of critical illness may have a similarly significant impact on functional outcomes and health care utilization.1,78 The parallels are striking.
There is considerable evidence that increased cytokine levels, in combination with reduced growth factor levels, contribute to sarcopenia and age-related decline. Early work showed an association between elevated IL-6 levels and advancing age where the highest levels were associated with the greatest degree of physical debility and significant mortality.79 Giresi and others use microarray gene expression profiling to identify genes that are dysregulated in older muscle, and specifically, appear to be upregulated by inflammatory factors.80
Recently, the importance of TNF-α has been highlighted in this literature. Higher levels of TNF-α and IL-6 have been associated with increased mortality in the community-dwelling elderly, a lower observed quadriceps strength in older men and women and stimulation of apoptotic signaling pathways.81 There appears to be a very complex interplay between these mediators and there may be some valuable, and potentially clinically applicable, insights as well. For example, IL-6 is released from skeletal muscle during exercise and this increase can result in an inhibition of TNF-α.82 It is possible that the benefits of early mobility programs, currently under study in many ICUs, not only address disuse atrophy but may also have important immunomodulatory effects on recovering skeletal muscle after critical illness.83-85
The collapse of these different risk strata into a single population or cohort for evaluation may account for the observed heterogeneity in functional outcomes currently reported in the literature and may obscure the ability to identify distinct clinical phenotypes. Risk modification is also an important consideration and deserves mention. Modifiers may include mood disorders,9,86 cognitive dysfunction,87,88 financial and family caregiver resources.
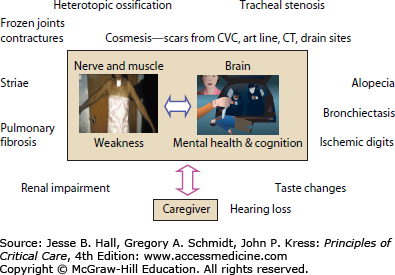
ICU survivors with ICUAW rely on family caregivers for support as they transition to home and reintegrate into the community. Approximately 57% of ICU survivors who received long-term mechanical ventilation still required the assistance of a family caregiver 1 year after their critical illness.89 Current literature suggests this may have a negative impact on caregivers, including poor HRQoL compared with age- and sex-matched persons,8 posttraumatic stress disorder,90 emotional distress,91-93 burden,94 depression,92 and anxiety.93 Previous work from our group found ARDS survivors’ depression and provision of high levels of care to be important contributors to caregiver depression.8 Others recently report caregivers experience more depression and difficulty maintaining participation in valued activities when caring for male ICU survivors with poorer functional ability.91,95,96 Determining the impact of ICUAW specifically on family caregiver health and well-being is necessary to understand the interplay between the survivor and the caregiver and the impact on recovery.
ADDITIONAL PHYSICAL MORBIDITIES
As discussed previously, the main morbidities of critical illness include ICUAW and neuropsychological dysfunction. However, several other physical sequelae also influence physical HRQoL and subsequent health care utilization. Again, these have been studied most extensively in survivors of ARDS. These include pulmonary dysfunction, entrapment neuropathy, late tracheal stenosis, heterotopic ossification, and a variety of cosmetic changes that have been linked to emotional outcomes, social isolation, and sexual dysfunction (Fig. 15-3).
Many ARDS survivors have persistent pulmonary function impairments that are typically mild restrictive changes and an associated reduction in diffusion capacity.1,78 Orme and colleagues reported that ARDS survivors had abnormal pulmonary function associated with decreased HRQoL 1 year following hospital discharge26 and Schelling et al reported no additional improvement in pulmonary function after the first year following ARDS.97 Neff and colleagues reviewed 30 studies that evaluated pulmonary function in ARDS survivors98 and found significant variability in the proportion of patients with obstructive (0%-33%) and restrictive (0%-50%) defects as well as compromised diffusion capacity (33%-82%). Most recent data from 5-year outcomes after ARDS show normal to near normal pulmonary function achieved by 6 months to 1-year after ICU discharge with continued stability over the 5-year study period. Evaluation of detailed chest imaging in these patients also showed minimal structural change to the pulmonary parenchyma in the majority of patients at 5 years after ICU discharge.99 One important limitation in this dataset was that 40% of patients were followed in their homes and therefore volumetric and diffusion capacity data were unavailable and some patients declined radiologic imaging at 5-year follow-up. This spectrum of pulmonary dysfunction may relate to population heterogeneity with respect to evolving definitions or severity of ARDS, severity of lung injury, ICU ventilatory strategy, prior history of lung disease or smoking, and the presence of other pulmonary processes that fulfill the ARDS definition but that have a very different natural history (eg, bronchiolitis obliterans organizing pneumonia). Most outcome studies found ARDS survivors are often unable to resume their prior physical function, but the degree of pulmonary dysfunction does not explain this degree of functional limitation.
The Toronto ARDS Outcomes study observed a 6% prevalence of peroneal and ulnar nerve palsies.1 Although this represents only a small proportion of patients, these nerve palsies complicated rehabilitation therapy and precluded return to original work in some cases. Clavet and colleagues highlighted the important contribution to disability made by the development and persistence of contractures during an episode of critical illness.100
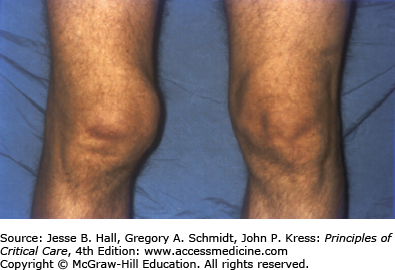
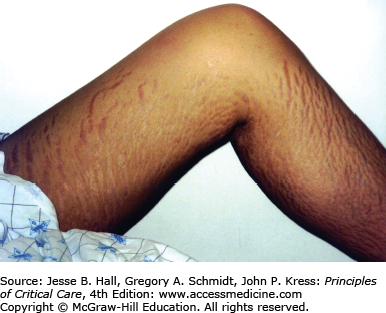
Heterotopic ossification is the deposition of paraarticular ectopic bone and has been previously associated with polytrauma, burns, pancreatitis, and ARDS.101 Heterotopic ossification is linked with paralysis and prolonged immobilization. There was a 5% prevalence of heterotopic ossification in the Toronto ARDS cohort study with all patients having large joint immobilization, leading to important functional limitation1 (Fig. 15-4). Heterotopic ossification is remediable with appropriate surgical intervention and screening for this may help to improve long-term function.
The physically transformative nature of critical illness cannot be overstated. Many patients suffer from the often devastating emotional effects related to their altered appearance. From 5-year outcomes data in ARDS survivors,2 many patients had ongoing concerns about cosmesis including scars from laparotomy, chest tube, central line, arterial line and tracheostomy insertion, burns, striae from volume overload, and facial scars from prolonged noninvasive mask ventilation (Fig. 15-5). Many patients underwent tracheostomy revision. Patients emphasized that cosmetic concerns contributed to social isolation and sexual dysfunction.
REHABILITATION FRAMEWORK—INTERNATIONAL CLASSIFICATION OF FUNCTIONING, DISABILITY, AND HEALTH102
The International Classification of Functioning, Disability, and Health (ICF) construct emphasizes disability and morbidity as the central determinants of health status. This is relevant if we consider that disability after critical illness is etiologically neutral. The ICF differs fundamentally from the International Classification of Disease (ICD), which focuses on disease as a cause of death. The ICF (Fig. 15-6) has three constituent parts, body function and structures, activity, and participation, and offers a comprehensive and multidimensional approach for rehabilitation interventions. This framework serves to highlight the interdependence of factors that should be highlighted and integrated into long-term and multimodality rehabilitation program.
The body functions and structures component of the ICF refers to physical function and/or specific organ system injury where impairment is characterized as any deviation from normal. Activity refers to the ability to execute important activities and participation refers to ability to conduct daily activity and “participation restriction” denotes difficulties experienced by patients when trying to carry out daily tasks. The constituent parts of the ICF capture function and disability and interact with the health condition, as well as personal and environmental factors. This is an important departure from constructs of quality of life that are personalized and capture a global functioning perspective based in feelings or satisfaction. For example, the generic short form-36 evaluates functioning in a disease context and does not incorporate aspects of patient participation or personal factors or environmental influence on outcome.
BARRIERS TO CONSTRUCTION OF REHABILITATIVE MODELS AFTER CRITICAL ILLNESS