FIG. 24.1 Transverse section of a peripheral nerve. (From Berde CB, Strichartz GR: Local anesthetics. In Miller RD, ed: Miller’s anesthesia, ed 8, Philadelphia, PA, 2015, Elsevier.)
Classification of peripheral nerves is important in determining the sequence of local anesthetic blockade. B fibers are the most sensitive. Dilation of cutaneous blood vessels is often the first sign of local anesthetic onset. C fibers and A-δ are next in sensitivity. They result in the inability to feel cool sensations such as an alcohol wipe. Next in sensitivity are the A-γ, A-β, and A-α fibers, which result in the loss of sensation, pressure, proprioception, and finally motor paralysis. Local anesthetic concentration must be adequate to block nerve fibers. It takes approximately twice the concentration of local anesthetic to block motor fibers as it does to block sensory fibers, and sympathetic fibers require the least. The order of blockade onset is B fibers > C fibers = A-δ fibers > A-γ fibers > A-β fibers > A-α fibers.
Table 24.1
Classification of Nerve Fibers
| Fiber Type | Myelin | Function |
| A-α | +++ | Motor (efferent: to skeletal muscle) |
| A-β | ++ | Touch, pressure, proprioception (afferent: from skin) |
| A-γ | ++ | Motor (efferent: to muscle spindles) |
| A-δ | ++ | Pain (sharp, fast) and temperature (efferent: from skin) |
| B | + | Preganglionic sympathetic (efferent: to vascular smooth muscle) |
| C | None | Pain (dull, slow) and temperature (afferent from skin); postganglionic sympathetic (efferent: to vascular smooth muscle) |
From Schick L, Windle PE, eds: Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing, ed 3, St. Louis, MO, 2015, Elsevier.
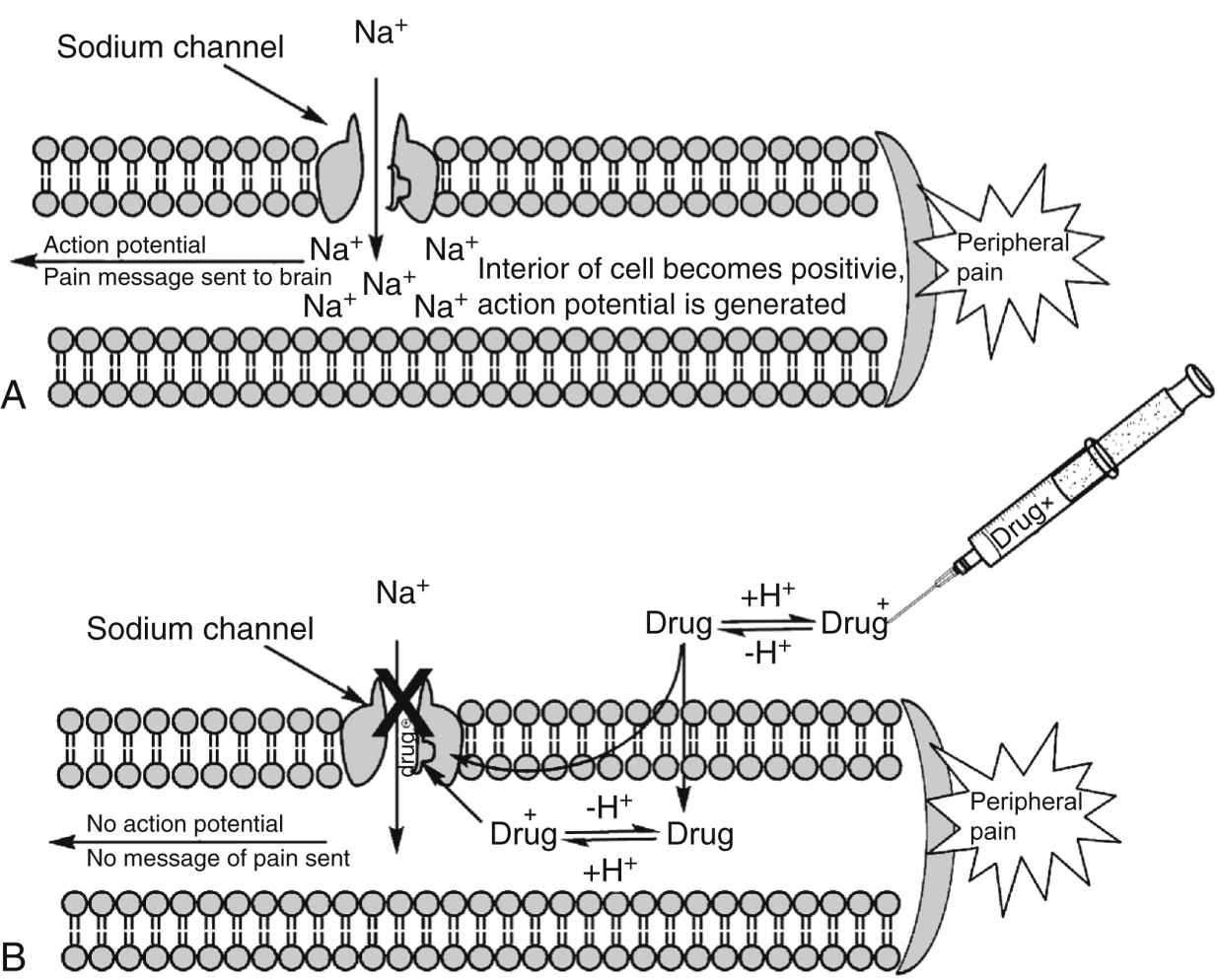
FIG. 24.2 Mechanisms of action for local anesthetics. A, Diagram of the axon of a sensory nerve cell transmitting the pain message to the CNS via depolarization of the nerve cell. B, Local anesthetic drugs, in their ionized form, bind to the sodium channel and prevent sodium from entering the nerve cell. The action potential is thus blocked, and the message of pain is not sent to the brain. (From Friel CJ, et al: Local anesthetic use in perioperative areas, Perioperative Nurs Clin 5:203–214, 2010.)
Recovery from local anesthetic happens in the opposite manner. It should be noted, however, that recovery usually occurs unevenly— that is, the patient may recover some motor and sensory functions simultaneously in different blocked areas. Different regional blocks may also exhibit different recovery patterns. The typical sequence of nerve blockade onset is shown in Box 24.1.3,4,6,8
Local Anesthetic Pharmacology
Chemistry and Stereoisomerism
A local anesthetic molecule contains a lipophilic group, an intermediate bond, and a hydrophilic group. Classification is dependent on the intermediate bond, which is either an ester (–CO–) or an amide (–HNC–). The lipophilic (attracted to fat) and hydrophilic (attracted to water) portions of the molecular structure are crucial to its ability to cross tissue barriers and reach their site of action. The intermediate bond, either an ester or an amide, determines the type of local anesthetic, affecting metabolism and potential for allergic reactions.4 Commonly administered amides and esters are noted in Box 24.2. Some differences between esters and amides are noted in Table 24.2.
Pharmacokinetics
Pharmacokinetics is the study of a medication’s absorption, distribution, metabolism, and excretion.6 Local anesthetics differ from most other drugs in that the perianesthesia nurse tries to keep them in the local tissue where they were injected. Once they are systemically absorbed they wear off. Systemic absorption can also lead to toxicity if too large a dose is given or if it is accidentally injected intravascularly. Fig. 24.3 depicts the fate of a local anesthetic once it is injected into a tissue.
Potency and duration of action of a local anesthetic are closely correlated to lipid solubility. In general, the more lipid soluble the local anesthetic, the more potent it is and the longer its duration of action. Potent local anesthetics readily bind with proteins and neural tissue leading to their longer duration of action and toxicity potential. Bupivacaine, ropivacaine, and tetracaine are the most potent. Lidocaine, mepivacaine, and prilocaine are moderate, whereas procaine and chloroprocaine are the least potent.4,7,8,9
Table 24.2
Clinical Differences Between the Ester- and Amide-Type Local Anesthetics
| Esters | Amides |
| Ester metabolism is catalyzed by plasma and tissue cholinesterase via hydrolysis; occurs throughout the body and is rapid. | Amides are metabolized in the liver by CYP1A2 and CYP3A4, and thus a significant blood level may develop with rapid absorption. |
| Although local anesthetic allergy is uncommon, esters have a higher allergy potential, and if patients exhibit an allergy to any ester drug, all other esters should be avoided. | Allergy to amides is extremely rare; there is no cross-allergy among the amide class or between the ester and amide agents. |
| Ester drugs tend to be shorter acting due to ready metabolism; tetracaine is the longest acting ester. | Amides are longer acting because they are more lipophilic and protein bound and require transport to the liver for metabolism. |
(From Nagelhout JJ, Elisha S: Nurse Anesthesia, ed 6, St. Louis, MO, 2017, Elsevier.)
Absorption of local anesthetics is dependent on site of injection, dose, vasoconstrictor, pharmacologic profile, and individual physiologic factors.3,4,10 Vascularity of tissue has a direct effect on systemic absorption (increases uptake and may lead to toxicity). Absorption of local anesthetic varies by route of administration. Absorption rates from highest to lowest are as follows: intravenous, tracheal, intercostal, caudal, epidural, brachial plexus, sciatic, and subcutaneous.3,10,11 Total dose is also a significant factor. The greater the dose, the greater the systemic absorption.6 Attention to the total dose is important to avoid toxicity. Epinephrine can be added to decrease systemic absorption, prolong duration of action through local vasoconstriction, and reduce toxicity. The ability of epinephrine to decrease systemic absorption varies by local anesthetic and injection site. The addition of epinephrine for peripheral nerve blocks and epidurals increases duration of action and decreases blood levels by 10% to 30% for lidocaine, mepivacaine, and bupivacaine but does not alter duration or blood levels for ropivacaine. Attention should be placed on the total dose of epinephrine to avoid deleterious effects.4,6 All local anesthetics, with the exception of cocaine and ropivacaine, cause a degree of vasodilatation. Vasodilation results in increased blood flow to the area of injection and absorption.
A final factor in determining the absorption rate of local anesthetics is individual physiologic conditions. Extremes of age are a factor because newborns have immature hepatic function, and the elderly have decreased hepatic enzyme function and blood flow, resulting in increased blood concentrations. During pregnancy, doses of local anesthetic should be decreased by one third because of hyperdynamic circulation and hormonal changes that can result in toxicity. Renal, hepatic, and cardiovascular disease can also affect local anesthetic pharmacokinetics.4,6,12
Uptake of local anesthetics results in their distribution to various tissues. The initial rapid disappearance phase (α) includes uptake into highly perfused tissue (brain, lung, liver, kidney, and heart). The lungs act as a large reservoir and decrease initial blood concentrations. The slow phase of disappearance (β) includes distribution to the gut and muscle. Muscle provides a large reservoir for local anesthetics.4,6,12 During pregnancy, amide local anesthetic molecules can be transferred across the placenta and accumulate in an acidotic environment; this is known as ion trapping.3,4
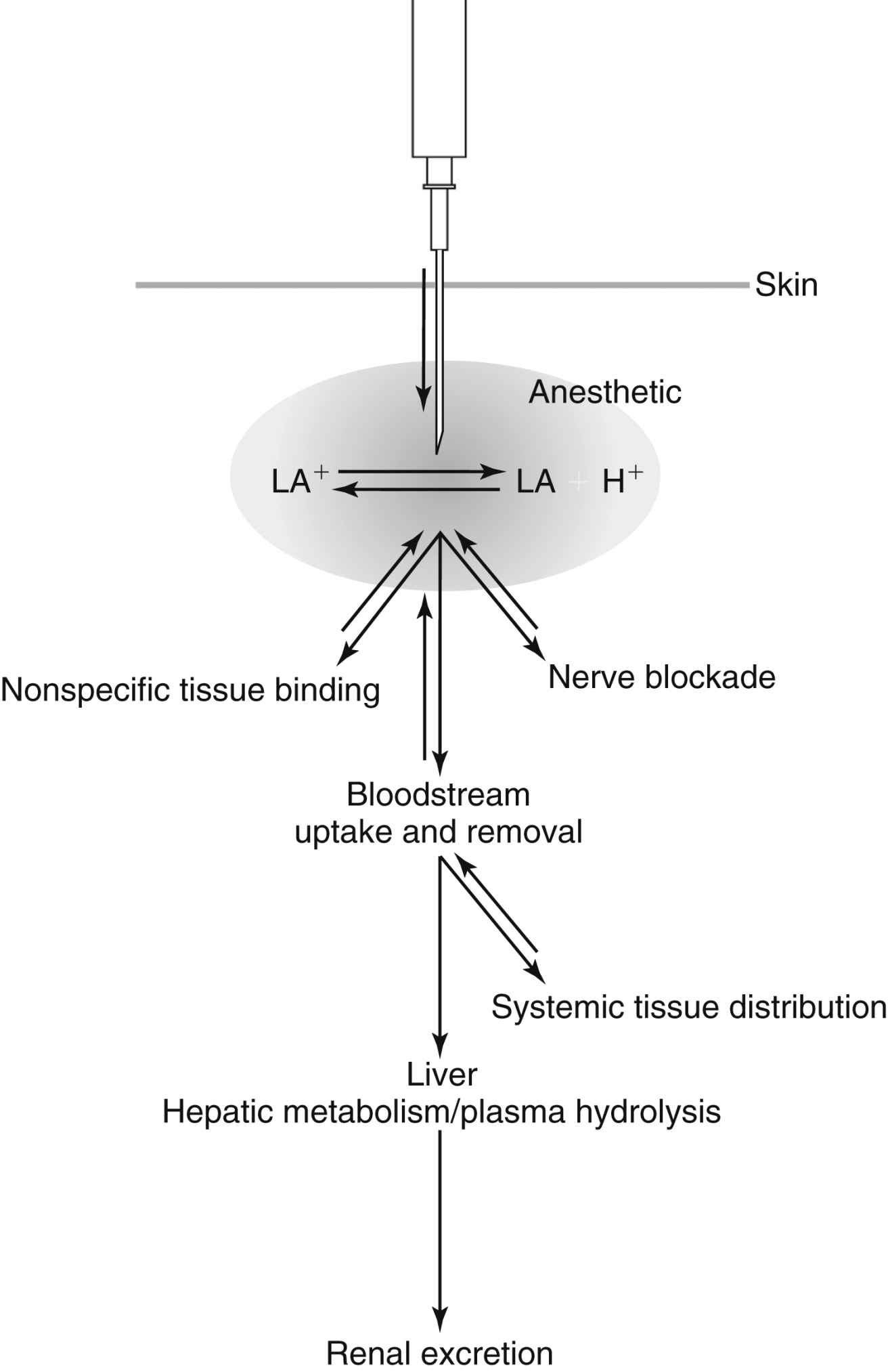
FIG. 24.3 Representation of the fate of a local anesthetic injected into tissue. (From Nagelhout JJ, Elisha S: Nurse Anesthesia, ed 6, St. Louis, MO, 2017, Elsevier.)
Metabolism depends on classification: amide or ester. Amides are metabolized primarily in the liver through the cytochrome P-450 system. Extremes of age and disease processes that affect liver perfusion and enzyme activity alter hepatic elimination of amides. Individual amides exhibit different clearance rates (prilocaine > lidocaine > mepivacaine > bupivacaine > ropivacaine). Esters undergo rapid hydrolysis in the blood, which limits toxicity. Plasma cholinesterase is the primary enzyme responsible.4 Caution should be used in patients with an acquired or genetic pseudocholinesterase deficiency, which can decrease metabolism and lead to potentially toxic blood concentrations.4,13 Metabolism of some ester local anesthetics results in the production of p-aminobenzoic acid (PABA), a known allergen. It is this byproduct of metabolism that makes ester local anesthetics more likely than amides to result in an allergic reaction.4,12
Maximum Doses
Maximum doses are general guidelines derived from animal studies in an attempt to identify median effective and toxic doses. Health care providers should take into account the site of injection, the addition or absence of epinephrine, and individual medical conditions such as age; renal, hepatic, and cardiac disease; and pregnancy.4 Refer to Table 24.3 for maximum doses of local anesthetics.
Individual Local Anesthetics
Refer to Table 24.3, which contains a summary of local anesthetic information.
Topical Anesthetics
Benzocaine is an ester local anesthetic that exists as a weak acid. It is used only as a topical local anesthetic. Onset is rapid, duration is short, and the maximum dose is 200 to 300 mg. Benzocaine is the most commonly implicated local anesthetic in methemoglobinemia. When using benzocaine, it is important that the manufacturer’s recommendations are strictly followed and patients are monitored for this complication.12 Eutectic mixture of local anesthetics (EMLA) cream is a mixture of 2.5% prilocaine and 2.5% lidocaine that can be applied topically to decrease the discomfort associated with venipuncture or incision, and it is particularly useful when starting intravenous lines on children. Dosing is based on patient weight and surface area, and manufacturer dosing recommendations require strict adherence. Application should occur on intact skin because absorption may be rapid with breaks in the integument. Onset is slow, and depth of penetration is limited. EMLA should not be used in infants younger than 3 months, infants 3 to 12 months old receiving additional medications that may predispose them to methemoglobinemia (i.e., nitrates, dapsone, sulfonamides, benzocaine), and children who may be predisposed to hereditary or congenital methemoglobinemia.4,6,10 Commonly used topical anesthetics are often a mixture of individual agents. Some current topical agents are listed in Table 24.4.
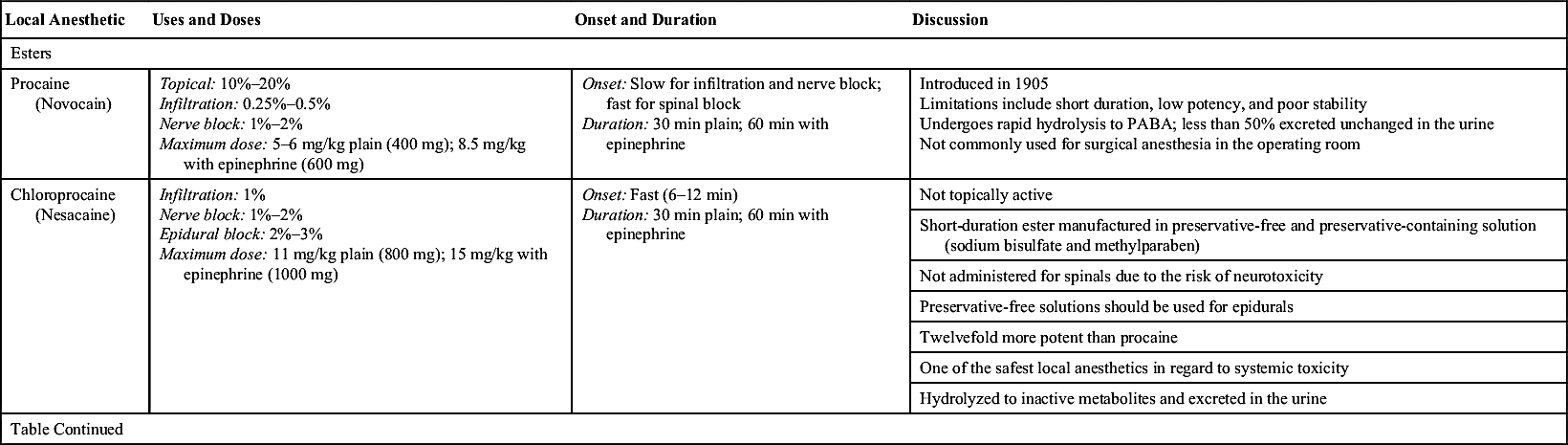
Table 24.3
Local Anesthetic Agents: Esters and Amides
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







