(1)
Trauma and Critical Care, R Adams Cowley Shock Trauma Center, Baltimore, MD, USA
Keywords
LiverHepatic injuryExsanguinating hemorrhageDiagnostic peritoneal lavage (DPL)Nonoperative management (NOM)American Association for the Surgery of Trauma Organ Injury Scale (AAST-OIS)Liver injury scalePringle maneuverAngiographic embolization (AE)Hepatobiliary iminodiacetic acid (HIDA) scanEndoscopic retrograde cholangiopancreatography (ERCP)Introduction of the Problem
The liver is the largest organ in the abdomen and is frequently injured. Although minor or moderate injuries can be managed with simple observation, the extensive vascular supply of the liver makes severe liver injuries extremely challenging. Frequently, patients who require liver-specific operations have exsanguinating hemorrhage. Despite improvements in resuscitation, mortality of operative liver injuries remains approximately 50 % [1, 2], with mortality of up to 80 % for patients with juxtahepatic venous injuries [3–6]. Morbidity rates following operation for high-grade liver injuries are as high as 30–80 % depending on the grade of injury and types of complications [7–10].
History of Care
The treatment of injuries to the liver has undergone a revolution, from the first description of injuries to the liver in Greek and Roman mythology to Hogarth Pringle’s description of the “Pringle maneuver” in 1908 [11]. Since that time, the management of hepatic injuries has transformed from early laparotomy with definitive repair and resection to damage control techniques in unstable patients and nonoperative management (NOM) in hemodynamically stable patients [12–14]. Successful operative management of liver injury was first reported in the early seventeenth century [15]. Reports from both military and civilian traumas documented successful operative management of hepatic injury, but morbidity and mortality rates remained extremely high, as was true for all major hemorrhage prior to the era of modern medicine [16–20]. In 1965, Root first described the diagnostic peritoneal lavage (DPL) [21]. As operative management of patients sustaining abdominal trauma who had hemoperitoneum on DPL was considered standard of care, the incidence of liver injury dramatically increased as did the experience with operative management. In the early 1980s, introduction of computed tomography (CT) allowed selective management of liver injuries to emerge [22]. In 1989, Moore et al. introduced the American Association for the Surgery of Trauma Organ Injury Scale (AAST-OIS) for liver injury, which was updated in 1995 and allows for a stratified qualification of liver injuries that is used to both guide intervention and allow for scientific comparisons of hepatic trauma [23, 24]. Grades from I to VI are described. Grade I and II are minor, grade III are moderate, and grade IV and V injuries are major. Grade VI injuries are uniformly fatal hepatic avulsion. See Table 11.1.
Table 11.1
AAST-OIS liver injury scale.
Grade of injury | Type of injury | Description of injury |
|---|---|---|
I | Hematoma | Subcapsular, <10 % surface area |
Laceration | Capsular tear, <1 cm depth | |
II | Hematoma | Subcapsular, 10–50 % surface area; intraparenchymal, <10 cm diameter |
Laceration | 1–3 cm depth, <10 cm length | |
III | Hematoma | Subcapsular, >50 % surface area or expanding; ruptured subcapsular or parenchymal hematoma; intraparenchymal hematoma >10 cm or expanding |
Laceration | >3 cm depth | |
IV | Laceration | Parenchymal disruption involving >25–75 % of lobe or 1–3 Couinaud’s segments within a lobe |
V | Laceration | Parenchymal disruption involving >75 % of lobe or >3 Couinaud’s segments within a lobe |
Vascular | Juxtahepatic venous injury | |
VI | Vascular | Hepatic avulsion |
Successful NOM of liver injuries in pediatric patients was then adopted in adults, with many large series published in more recent years [25–29]. It was in 1908 that Pringle published the landmark study that not only first described the “Pringle maneuver,” but was also likely the first surgeon to advocate for NOM of low-grade injuries and stratify treatment options by severity of injury:
It is very probable that slight ruptures will occasionally heal without surgical interference in consequence of this increased tension of the abdominal wall leading to the arrest of hemorrhage, but in the cases of severe injury to the liver this will not happen. [11]
Although for low-grade injuries, NOM has been advocated for decades, there was considerable controversy about the management of high-grade injuries. Studies by Croce and Pachter in the mid-1990s convincingly demonstrated that high-grade injuries could be managed safely [25, 26]. Both found that NOM is safe for hemodynamically stable patients regardless of injury severity and/or amount of hemoperitoneum. The current paradigm is that hemodynamics alone should dictate which patients require immediate operative intervention for hepatic trauma [30].
Technique with Personal Tips
Knowledge of vascular anatomy—the hepatic arteries, the portal venous system, and the hepatic veins—is essential to manage hemorrhage while preserving hepatic function in complex hepatic injury. Familiarity with the anatomic relationships and preservation of main conduits of the biliary tree is also essential (Fig. 11.1). The classic description of the liver anatomy is based on external appearance in which the falciform ligament extends from the diaphragmatic surface to the abdominal wall and divides the liver into the right and left anatomic lobes. The liver is most commonly described by the eight Couinaud’s segments, which are dictated by the vascular anatomy rather than external landmarks [31] (Figs. 11.2 and 11.3). This classification is more useful for operative liver management, since it divides the liver into units based on the key structures essential for safe operative technique. The center of each Couinaud’s segments contains the supplying branch of the portal vein and hepatic artery running with the bile duct while hepatic veins lie in the periphery of each segment. A functional left and right liver is divided by a main portal fissure known as Cantlie’s line, which runs from the middle of the gallbladder fossa to the inferior vena cava (IVC) and contains the middle hepatic vein. The portal vein divides the liver into upper and lower segments by the left and right portal veins, which then branch superiorly and inferiorly. The hepatic arteries run with the portal veins as do the main biliary ducts. The hepatic venous drainage includes the right hepatic vein, which divides the right lobe into anterior and posterior segments; the middle hepatic vein, which divides the liver into right and left lobes in a plane along Cantlie’s line; and the left hepatic vein, which divides the left lobe into a medial and lateral part. The caudate lobe, segment I, is drained by one or more short hepatic veins that drain directly into the IVC. Another key consideration is the external hepatic artery anatomy, which can be aberrant in 25 % of patients [32]. The most common of these is the “replaced right hepatic artery” in which the right hepatic artery originates from the superior mesenteric artery rather than the proper hepatic artery, which is an important consideration when dissecting out the porta hepatis and/or applying a “Pringle maneuver.”
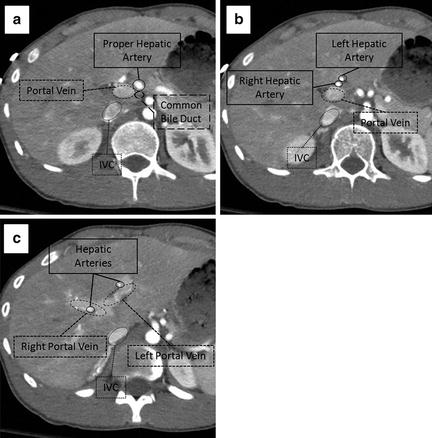
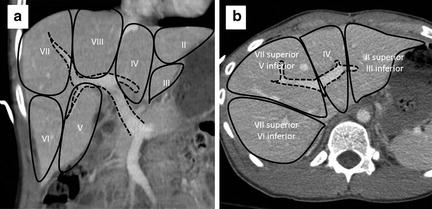
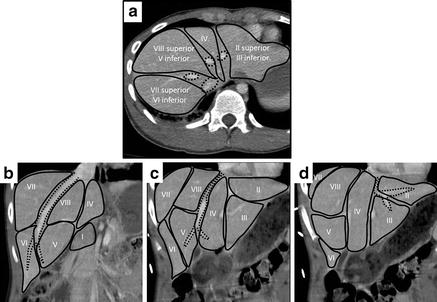

Fig. 11.1
Hepatic anatomy. (a) The relationship of the portal vein (dashed line), proper hepatic artery (solid line), and common bile duct (large dashed line) in the porta hepatis. (b) The relationship of the portal vein (dashed line) and right and left hepatic arteries (solid lines). (c) Bifurcation of the portal vein (dashed line) separating segments 8 (superiorly) and 5 (inferiorly) on the right from segment 4 on the left. The main branches of the hepatic arteries (solid line) can be seen running with the portal vein.

Fig. 11.2
Portal venous anatomy (dashed line) dividing the liver into Couinaud’s segments. Segment I (not shown) is the caudate lobe. (a) Coronal view. (b) Axial view.

Fig. 11.3
Hepatic venous anatomy (dotted line) dividing the liver into Couinaud’s segments. Segment I is the caudate lobe. (a) Axial view. Dotted circles indicate IVC, right, middle, and left hepatic veins (clockwise from bottom). Coronal views showing the right hepatic vein (b), middle hepatic vein (c), and left hepatic vein (d) and relationships to the functional hepatic segments.
Operative management of liver injuries typically occurs in one of two settings:
1.
The patient is being explored for both diagnosis and therapy—most typically in the situation of hemodynamic instability, peritonitis, or a transperitoneal penetrating trajectory.
2.
The patient is being explored for another indication such as bowel or bladder injury, and a liver injury is encountered.
In the first situation, a standard midline trauma exploratory laparotomy should always be performed with initial packing (Fig. 11.4). Exsanguinating hemorrhage should be anticipated, and activation of a massive transfusion protocol, availability of rapid transfusion systems, and careful coordination with the anesthesia providers are essential. The anterior portion of the falciform ligament is taken down between hemostats and tied to minimize risk of bleeding from a patent remnant umbilical vein. At this point in the operation, the falciform ligament should not be taken down any farther posteriorly than required to gain adequate access to the peritoneal cavity to minimize the risk of release of retrohepatic tamponade. Packing around the liver typically includes three laparotomy pads (“lap pads”) above and below the liver to compress the liver up against the diaphragm (Fig. 11.5). Even at this early stage in the operation, significant liver injuries should be readily apparent. There are two crucial questions that need to be answered:
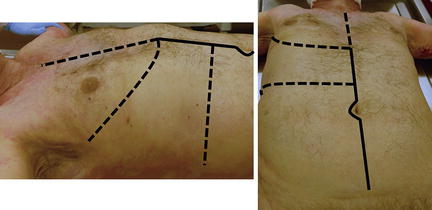
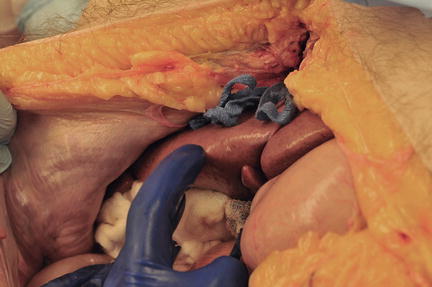

Fig. 11.4
Exposure for operative management of liver injuries. Standard midline laparotomy (solid line). Additional incisions for exposure when needed (dashed line). Photo credit: Facilities, Surgical Laboratory, Anatomical Services Division, School of Medicine, UMB; Cadavers and Specimens, Anatomical Donors, Maryland State Anatomy Board; Ronn Wade, State Anatomy Board, Department of Health and Mental Hygiene.

Fig. 11.5
Simple “lap pad” packing of the liver. Typically three lap pads are packed above and three lap pads below the liver for temporary hemostasis. Photo credit: Facilities, Surgical Laboratory, Anatomical Services Division, School of Medicine, UMB; Cadavers and Specimens, Anatomical Donors, Maryland State Anatomy Board; Ronn Wade, State Anatomy Board, Department of Health and Mental Hygiene.
1.
Is the patient bleeding through packs?
2.
Does the patient’s physiology dictate that a damage control (DC) approach should be used?
If the answer to question #1 is “no,” then time is given to allow the anesthesia providers to administer blood products and stabilize the patient’s hemodynamics. Definitive repair and hemostasis is then dictated by the answer to question #2. If the patient is physiologically normal, then definitive hemostasis and repair can be performed as discussed below. If a DC approach is advisable and the patient is not actively bleeding with the lap pads in place, no further liver-specific intervention is indicated at that time and other active issues can be addressed such as control of enteric contamination.
If the answer to question #1 is “yes,” then a series of maneuvers should be performed in an orderly and sequential fashion (Fig. 11.6). The right upper quadrant should be unpacked and inspected. Manual compression is the first-line therapy for all hemorrhage from the liver and is surprisingly effective. While the assistant is compressing the liver parenchyma, the liver must be fully mobilized. The exception to this is in patients with retrohepatic hematoma that appears to be stable. In this situation, full mobilization of the liver may result in release of tamponade from bleeding from a major hepatic venous injury or retrohepatic caval injury. All other injuries are best approached with full mobilization of the liver (Fig. 11.7). The liver is mobilized by taking down the falciform ligament all the way to the suprahepatic IVC using either electrocautery or Metzenbaum scissors. The hepatic veins are not encountered until the leaflets of the falciform ligament diverge so the ligament can be dissected all the way back to the IVC with impunity. The right and left triangular ligaments are similarly transected until the liver can be mobilized out of the right upper quadrant and inspected. This should be done extremely rapidly and take only a minute or two to completely mobilize the liver. At this point the liver can be fully inspected, and the major source of hemorrhage can be identified.
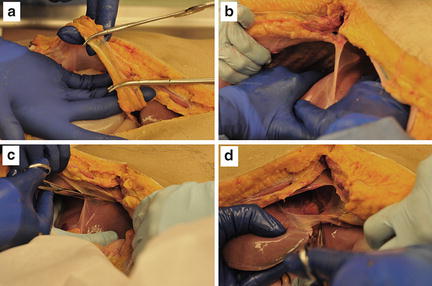

Fig. 11.6
Algorithm for initial operative management of major hepatic injury. DC = damage control, DH = definitive hemostasis, THI = total hepatic isolation.

Fig. 11.7
Mobilization of the liver. (a) The anterior portion of the falciform ligament is clamped and tied. (b, c) The liver is retracted downward and the falciform ligament is sharply transected back to the IVC. (d) The right (not shown) and left triangular ligaments are similarly transected. Photo credit: Facilities, Anatomical Services Division, School of Medicine, UMB; Cadavers and Specimens: Anatomical Donors, Maryland State Anatomy Board; Ronn Wade, State Anatomy Board, Department of Health and Mental Hygiene.
If active hemorrhage is still encountered that cannot be controlled with simple manual pressure, a “Pringle maneuver” is next applied (Fig. 11.8). The gastrohepatic ligament is opened, taking care to avoid injury to the left gastric artery, and the porta hepatis is encircled through the foramen of Winslow. A vascular clamp or Penrose drain can then be used to compress the portal vein and proper hepatic artery at the porta hepatis. This maneuver controls all but hepatic venous flow, so if the hemorrhage from the liver is stemmed, a major hepatic venous injury is unlikely. Decisions about definitive hemostasis for a hepatic arterial or portal venous injury can then be made. If massive exsanguination continues, then the diagnosis of hepatic venous injury is likely and the techniques described next will need to be rapidly employed. If the majority of the bleeding appears to be from the posterior aspect of the liver, a major hepatic vein or retrohepatic caval injury is likely.
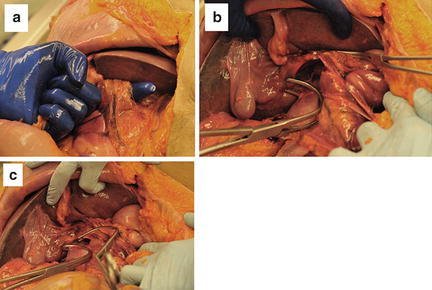

Fig. 11.8
(a) Dissecting out the porta hepatis for a “Pringle maneuver.” (b) Exposure to the infrahepatic IVC. (c) Infrahepatic IVC occlusion with the portal triad occluded. Photo credit: Facilities, Surgical Laboratory, Anatomical Services Division, School of Medicine, UMB; Cadavers and Specimens, Anatomical Donors, Maryland State Anatomy Board; Ronn Wade, State Anatomy Board, Department of Health and Mental Hygiene.
If a major hepatic vein or retrohepatic caval injury is suspected, additional exposure is advisable. There are several strategies for this. Access to the hepatic veins and IVC requires a more extensive mobilization and, in our opinion, is best accomplished by opening the thorax. The best exposure will be obtained by extending the midline laparotomy incision across the right chest at about the eighth intercostal space and transecting the costal cartilages (Fig. 11.4). The diaphragm is then taken down from the anterior midline radially all the way back to the IVC, remembering to leave enough diaphragm on the chest wall side of the incision for later repair (Fig. 11.9). At this point a chest retractor can be placed and exposure to the entire retrohepatic area is achieved (Fig. 11.10). The other strategy that can be used to gain additional exposure to the liver, particularly the posterior right lobe and retrohepatic area, is to “t-off” the laparotomy incision through a right lateral transverse incision in which the rectus muscles are transected (Fig. 11.4). This allows for improved access to these structures and facilitates mobilization and the ability to apply direct circumferential pressure to the liver parenchyma.
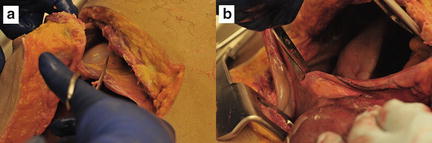
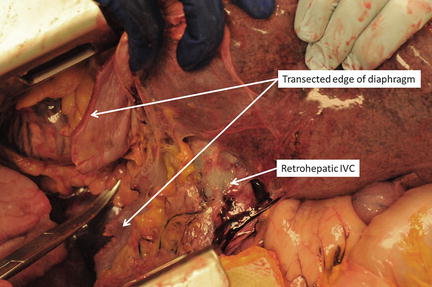

Fig. 11.9
Extension of the midline laparotomy to the right chest at the eighth intercostal space and transecting the costal cartilages. The diaphragm is then taken down from the anterior midline (a) radially all the way back to the IVC (b), remembering to leave enough diaphragm on the chest wall side of the incision for later repair. Photo credit: Facilities, Surgical Laboratory, Anatomical Services Division, School of Medicine, UMB; Cadavers and Specimens, Anatomical Donors, Maryland State Anatomy Board; Ronn Wade, State Anatomy Board, Department of Health and Mental Hygiene.

Fig. 11.10
Retrohepatic IVC exposure after extension of the midline laparotomy into the right thorax. Photo credit: Facilities, Surgical Laboratory, Anatomical Services Division, School of Medicine, UMB; Cadavers and Specimens, Anatomical Donors, Maryland State Anatomy Board; Ronn Wade, State Anatomy Board, Department of Health and Mental Hygiene.
Although classically taught, the technique of total hepatic isolation is, in our opinion, not as effective or as straightforward as typically described. Additionally, the profound reduction in preload that occurs with clamping of the suprahepatic IVC in a patient who already is in shock often leads to cardiac arrest. If total hepatic isolation is desired, however, once the chest is open, the suprahepatic IVC can be easily clamped by simple incision of the right lateral pericardium, taking care to avoid injury to the phrenic nerve (Fig. 11.11). Alternative access to the suprahepatic IVC without thoracic exposure can be obtained via gentle traction down on the dome of the liver with upward traction on the diaphragm and intraperitoneal dissection and exposure. An incision through the peritoneal side of the diaphragm into the pericardium and clamping of the suprahepatic IVC in the pericardium is another approach to suprahepatic IVC control (Fig. 11.12). A median sternotomy also provides immediate access to the suprahepatic IVC. The infrahepatic IVC is accessed either through the gastrohepatic ligament or, more commonly, via a complete right medial visceral rotation with a Kocher maneuver. The IVC is carefully encircled above the renal veins and clamped with a vascular clamp (Fig. 11.8). With the porta hepatis, the suprahepatic IVC, and the intrahepatic IVC now occluded, total hepatic vascular isolation is achieved. As stated previously, however, this is typically poorly tolerated by patients in extremis.
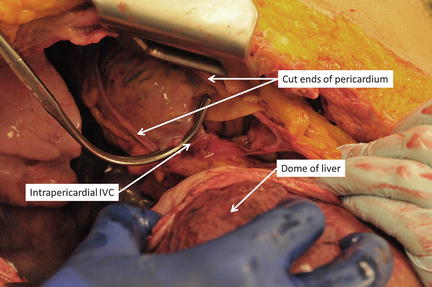
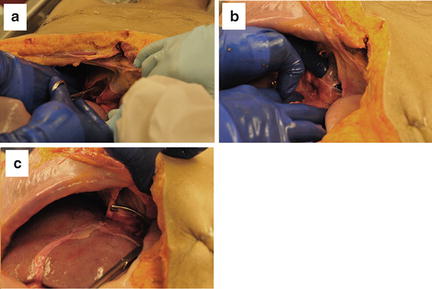

Fig. 11.11
Suprahepatic IVC clamped after exposure through the right thorax with incision in the pericardium. Photo credit: Facilities, Surgical Laboratory, Anatomical Services Division, School of Medicine, UMB; Cadavers and Specimens, Anatomical Donors, Maryland State Anatomy Board; Ronn Wade, State Anatomy Board, Department of Health and Mental Hygiene.

Fig. 11.12
Accessing the suprahepatic IVC. The pericardium is incised through the diaphragm (a), and the suprahepatic IVC is encircled (b) and clamped (c). Photo credit: Facilities, Surgical Laboratory, Anatomical Services Division, School of Medicine, UMB; Cadavers and Specimens, Anatomical Donors, Maryland State Anatomy Board; Ronn Wade, State Anatomy Board, Department of Health and Mental Hygiene.
The use of veno–venous bypass can allow for preservation of preload and venous return to the heart with total hepatic isolation [33, 34]. Venous cannulae can be placed percutaneously in the right internal jugular and right femoral veins allowing for preservation of flow to the heart. Additionally, this technique can be employed without the use of total hepatic isolation as a way to markedly decrease bleeding from the hepatic veins and the IVC to allow for better visualization and a reduction in blood loss during repair or ligation of these structures. Veno-venous bypass is exceptionally helpful when major resection is needed at planned “second-look” laparotomy or for management of major complications.
The atriocaval shunt was first described in 1968 by Schrock et al. as a strategy of controlling hemorrhage from retrohepatic IVC and hepatic venous injury [35]. The technique includes placement of a large-bore chest tube into the right atrium after a purse-string suture is placed in the atrial appendage. The side hole of the chest tube must be within the atrium to allow for maintenance of venous inflow. The tip of the tube is clamped, and the tube is then passed down into the IVC and secured at the intrapericardial and infrahepatic IVC with simple ties, Rummel tourniquets, or umbilical tape. When used with a Pringle maneuver, it should provide almost total hepatic vascular occlusion. Case series have reported survival in these highly lethal injuries [36, 37]. In 1986, Pachter reported six consecutive patients with juxtahepatic venous injuries managed without a shunt with a remarkable survival of 83 % [38]. While we are not big proponents of its use, the key to its successful employment is clearly the decision to utilize the technique early in the operation before the patient is moribund [36].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








