KEY POINTS
Patients are candidates for liberation from mechanical ventilation when gas exchange or circulatory disturbances which precipitated respiratory failure have been reversed.
More than half of all critically ill patients can be successfully liberated from mechanical ventilation after a brief trial of spontaneous breathing on the first day that reversal of precipitating factors is recognized. Gradual reduction of mechanical support, termed weaning, is frequently unnecessary and can prolong the duration of mechanical ventilation.
Once a patient has been liberated from the ventilator, extubation should follow if mechanisms of airway maintenance (cough, gag, swallow) are sufficient to protect the airway from secretions. Whether to extubate is a decision which follows successful liberation from the ventilator.
In patients who fail their first trial of spontaneous breathing, attention should turn to defining and treating the pathophysiologic processes underlying failure.
One weaning regimen, the gradual reduction of intermittent mandatory breaths, prolongs patients’ time on mechanical ventilation.
Liberation from mechanical ventilation is achieved most expeditiously if patients are given a trial of spontaneous breathing (T-Piece or pressure support ≤7 cm H2O) each day. Patients remain on ventilators unnecessarily when clinicians do not put this simple plan in place.
Patients who have had most correctable factors addressed and remain marginal with regard to ventilatory capacity should in most circumstances undergo a trial of extubation rather than remain intubated for protracted periods of time. Noninvasive positive pressure ventilation may be useful in these patients to transition them to fully spontaneous breathing following extubation.
Positive pressure ventilation can be lifesaving, but is also associated with many complications (Table 60-1). Most studies have demonstrated that earlier withdrawal of mechanical ventilatory support, when feasible, is associated with better outcomes. We will outline principles and approaches to the withdrawal of mechanical ventilation in a way to achieve this milestone at the earliest possible time and in a safe fashion.
Complications Associated With Endotracheal Intubation and Mechanical Ventilation
| Complications Related to the Endotracheal Tube |
| Endotracheal tube malfunction—mucus plug, cuff leak |
| Endotracheal tube malposition |
| Self-extubation |
| Nasal or oral necrosis |
| Pneumonia |
| Laryngeal edema |
| Tracheal erosion |
| Sinusitis |
| Complications Related to the Ventilator |
| Ventilator-induced lung injury (VILI) |
| Ventilator-induced diaphragm dysfunction (VIDD) |
| Alveolar hypoventilation/hyperventilation |
| Atelectasis |
| Hypotension |
| Pneumothorax |
| Diffuse alveolar damage |
| Effects on Other Organ Systems |
| Gastrointestinal hypomotility |
| Pneumoperitoneum |
| Stress gastropathy and gastrointestinal hemorrhage |
| Arrhythmias |
| Salt and water retention |
| Malnutrition |
LIBERATION STRATEGIES
Many intensivists have reasoned that by gradually reducing ventilatory support, the respiratory muscles exercise at subfatiguing loads, leading to gradual improvement of function. Some studies have suggested that respiratory exercises (repetitions of low-load resistive breathing) can lead to successful extubation in patients who have previously failed.1 However, no studies have established that respiratory muscle training, through the use of graded withdrawal of ventilatory support, hastens the recovery to unassisted breathing.
Two large studies assessed the role of weaning strategies once clinicians judge that weaning can proceed. Brochard and colleagues2 studied 456 medical-surgical patients being considered for weaning, of whom 347 (76%) were successfully extubated on the first day. One hundred and nine patients who failed an initial spontaneous breathing trial (SBT) were randomized to be weaned by one of three strategies: (1) T-piece trials of increasing length until 2 hours could be tolerated; (2) synchronized intermittent mandatory ventilation (SIMV) with attempted reductions of 2 to 4 breaths/min twice a day, until 4 breaths/min could be tolerated; (3) pressure support ventilation (PSV) with attempted reductions of 2 to 4 cm H2O twice a day until 8 cm H2O could be tolerated. Patients randomized to the three strategies were similar with regard to disease severity and duration of ventilation before weaning. There was no difference in the duration of weaning between the T-piece and SIMV groups, but PSV led to significantly shorter weaning compared to the combined T-piece and SIMV cohorts.
Esteban and colleagues3 performed a similar study of 546 medical-surgical patients, 416 (76%) of whom were successfully extubated on their first day. The 130 patients who failed were randomized to undergo weaning by (1) once-a-day T-piece trial, (2) two or more T-piece or CPAP trials each day as tolerated, (3) PSV with attempts at reduction of 2 to 4 cm H2O at least twice a day, and (4) SIMV with attempts at reducing 2 to 4 breaths/min at least twice a day. Patients assigned to the four groups were similar with regard to demographic characteristics, acuity of illness, and a number of cardiopulmonary variables. The weaning success rate was significantly better with once-daily T-piece trials than for PSV and SIMV. Twice-daily T-piece trials were not significantly better.
Several important conclusions can be drawn from these relatively large studies. First, and most important, the majority of patients can be successfully extubated on the first day that physicians recognize readiness after a brief (30-120 minute) trial of breathing through a T-piece: weaning is not necessary for most patients. Second, both studies suggest that in patients who have failed an initial T-piece trial, SIMV weaning prolongs the duration of mechanical ventilation.
That most patients can be extubated on the first day suggests that clinicians are slow to recognize that patients no longer require ventilatory support. In a landmark study, a daily screen and SBT were used to identify patients who had recovered from respiratory failure.4 Simply notifying physicians that patients had passed an SBT reduced the duration of ventilation by 1.5 days; lessened complications; and lowered costs. This was followed by another trial showing that a therapist-directed protocol to conduct SBTs, without daily supervision by a weaning physician, was feasible and safe.5
EXPEDITING LIBERATION
Since mechanical ventilation has numerous risks, including infection and barotrauma6-8 (see Table 60-1), it is appropriate to work aggressively to repair the “broken” patient; prevent new problems; and determine each day whether the patient still requires the ventilator. Many ICUs employ a “ventilator bundle,” including head-of-bed elevation; daily sedative interruption; breathing readiness assessment; and prophylaxes against thromboembolism and gastrointestinal hemorrhage to ensure attention to these important measures.9 We highlight methods used to expedite readying the patient for liberation and to identify patients who are appropriate candidates for liberation. We also describe an approach to patients who do not rapidly succeed at being liberated from mechanical ventilation.
In order to minimize the duration of ventilator dependence, the clinician must:
Identify the pathogenesis of respiratory failure in each patient, and institute appropriate treatment.
Prevent iatrogenic complications.
Detect when the patient is ready to breathe.
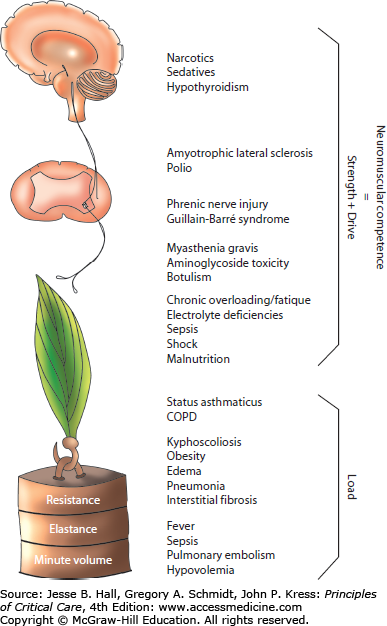
Although it may seem intuitive that an organized, systematic approach aimed at remedying the pathogenesis of disease should expedite liberation from mechanical ventilation, this has been examined rarely.10 A protocol that combined identifying and repairing causes of failure with recognizing readiness to breathe, reduced ventilator days and costs. From this study it is not possible to determine the relative importance of disease reversal and readiness assessment, but both are likely important. Accordingly, from the very first day a patient requires intubation it is worthwhile defining the mechanisms causing the need for mechanical ventilation (Fig. 60-1).
FIGURE 60-1
The neuromuscular circuit. This diagram summarizes the components of neuromuscular competence and respiratory muscle load and illustrates processes which can affect the strength-load balance leading to ventilatory failure. (Reproduced with permission from Manthous CA, Siegel M. Ventilatory failure. In: Matthay et al, eds. Pulmonary and Critical Care Yearbook, vol 3. St. Louis, Mosby; 1996, Chap. 2.)
Hypotheses regarding pathogenesis can be confirmed shortly after intubation by evaluating the chest radiograph, arterial blood gases, lung ultrasound, and ventilator pressure and flow waveforms as described in Chaps. 29 and 48. By discerning the causes of respiratory failure, the clinician can initiate appropriate treatments early and understand which parameters best reflect disease resolution.
Although not emphasized in most discussions of “weaning” from mechanical ventilation, strategies that avoid further injury during mechanical support are extremely important to ultimately returning the patient to spontaneous breathing. Such injuries can be characterized as those wrought directly by the ventilator, and those associated with being in the ICU. Ventilator-induced lung injury (VILI) (see Chap. 51) refers to a number of mechanisms by which lung injury is amplified in ARDS but can be produced in otherwise healthy lungs as well.11,12 Ventilator-induced diaphragm dysfunction (VIDD) describes the loss of respiratory muscle function related to mechanical ventilation and acute illness, and is discussed more fully in Chap. 49.13 Patients with severe airflow obstruction are at risk for dynamic hyperinflation and adverse consequences such as hypotension and diminished venous return (see Chap. 54).14
Indirect complications of mechanical ventilation include aspiration, which should be prevented by maintaining the head of the bed of all ventilated patients at 30° unless contraindicated.15 Ventilated patients are often sedentary for a substantial portion of each day and therefore at risk of deep venous thrombosis, justifying universal prophylaxis with pharmacologic therapies (preferred) or pneumatic compression devices (if anticoagulants are contraindicated).16 Gastric mucosal protection should be provided for ventilated patients.17 Protein pump inhibitors, H2-receptor blockers, sucralfate, and antacids have been used to prevent gastric injury. Whether continuous feeding of the gut, which usually neutralizes pH, obviates the need for prophylaxis remains unclear.
Arguably, one of the most important advances in care of critically ill, ventilated patients is realization that medications administered in the past to facilitate comfort can be harmful. Accumulating evidence suggests that sedatives and opiates promote a variety of neurocognitive complications. Most importantly, deep sedation prevents mobilization and there is now strong evidence that disuse atrophy and prolonged disability result when critically ill patients remain at bedrest and deeply sedated. Minimal use of such medications coupled with early physical therapy18,19 improves outcomes of mechanically ventilated patients, including fewer days of ventilation. Sedatives and opiates have been associated with other serious complications of critical illness including delirium,20-21 depression, posttraumatic stress disorder, and persistent cognitive deficits.22-25 When these medications are used, they should be used on a “PRN” basis26,27 titrated to the minimal amount to maintain a comfortable, arousable patient (Chap. 22). Continuous infusions of sedatives and opiates should be avoided whenever possible, as both classes of medications are fat soluble and may accumulate causing prolonged sedation. If continuously infused medications are used, a period of daily awakening improves outcomes.18,28 Sedative guidelines have been updated to reflect these new findings.27
One method of reducing the amount of sedative is to adjust ventilator settings in accord with patient comfort before resorting to large doses of sedatives and narcotics. Of course, this may not be possible, or may lead to settings that risk VILI or dynamic hyperinflation. Since patients’ respiratory status changes often, daily examination for comfortable ventilator settings may be useful as described in Chaps. 48 and 49.
Another complication of critical illness is fluid overload.29,30 It is not uncommon for survivors of critical illness to accumulate 10 or even 20 L of fluid prior to beginning the recovery process. When positive pressure is removed from the chest during spontaneous breathing, blood is centralized, so it is not unexpected that congestive heart failure is among the most common reasons for weaning failure. An accumulating body of evidence suggests that cumulative fluid balance is a determinant of the duration of ventilator dependence.29-31 Early initiation of fluid restriction targeting a central venous pressure of 4 cm H2O enhances outcomes of patients with ARDS.30 Much hypervolemia can be prevented by avoiding maintenance fluid infusions, accounting daily for the net fluid balance, limiting fluid boluses to patients likely to benefit from it (see Chap. 34), and using diuretics and renal replacement therapy before hypervolemia is excessive.
Inspiratory muscles suffer atrophy and contractile dysfunction during critical illness and mechanical ventilation.13,32 This VIDD is seen early, progresses quickly, and is associated with prolonged ventilation and death. In animal models, ventilator modes that maintain active contraction (assisted, rather than controlled, modes) largely prevent VIDD. These findings suggest that controlled modes should be avoided, when possible.
Weaning implies gradual, rather than rapid, withdrawal of ventilatory assistance. This word suggests that the ventilator is beneficial or nurturing and that the mechanism for successfully separating the patient is to gradually adjust the machine. Liberation more accurately describes the process by which most patients are freed from the ventilator. For many, this is as simple as recognizing that the ventilator is no longer needed. In patients who cannot breathe independently, liberation will only be possible after the patient is treated and recovers.
CAN THE PATIENT BREATHE WITHOUT THE VENTILATOR?
Historically “weaning parameters” were used to predict patients’ ability to breathe without the ventilator. However, despite decades of research, no weaning parameter has predictive accuracy sufficient to be used exclusively to make liberation decisions.33 Moreover, the question can be answered directly with a trial of spontaneous breathing (SBT). Accordingly, we do not use weaning parameters routinely to make liberation decisions.34
The use of interdisciplinary weaning teams4,35,36 or respiratory therapist- driven protocols may expedite successful liberation by actively addressing this question each day. Other studies have applied very different algorithms to achieve significant reductions in duration of ventilation.10,37 However, they all have one thing in common: they substitute a program of daily systematic scrutiny of readiness for breathing for the individual variation occurring in unstructured care systems. Whether achieved by protocol or by individual clinician perseverance, we believe that patients can be liberated from mechanical ventilation more expeditiously if they are screened on a daily basis.
Pressure support, continuous positive airway pressure (CPAP), and T-piece trials are the most common methods used to test readiness for liberation from mechanical ventilation. Strong evidence is lacking to support one approach over the others. An advantage of T-piece trials is simplicity, but some patients failing T-piece can safely be extubated.38 Most intensivists prefer 5 to 7 cm H2O pressure-support because this maintains the monitoring and alarming functions of the ventilator; this degree of ventilator assistance does not generally produce false negatives (ie, passing the SBT does not lead to excessive extubation failures; although patients with primary neuromuscular disease may be an exception); PEEP can be continued; and most large mechanical ventilation trials have employed this approach.
In preparation for the SBT, sedatives and narcotics should be discontinued several hours beforehand to reduce the likelihood of inadequate drive to breathe. Coordinating the sedative interruption and SBT improves success, reducing time on the ventilator and even long-term mortality.28 Especially when SBTs are conducted by protocol, a safety screen is necessary to reduce risk and select patients most likely to benefit. Typical safety screens require hemodynamic stability; adequate oxygenation on an acceptable PEEP; some spontaneous breathing effort; and absence of agitation, cardiac ischemia, or intracranial hypertension (Fig. 60-2). In individual circumstances and with appropriate monitoring, an SBT can be conducted despite higher than nominal levels of PEEP or while patients are still requiring vasoactive infusions for shock, since the ventilator may be a more noxious intervention than norepinephrine, for example.
FIGURE 60-2
A simple bedside algorithm for liberating patients from mechanical ventilation and performing a trial of extubation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree