Figure 13.1
Types of hiatal hernia (Illustration by Clinton T. Morgan, M.D.)
Preoperative Preparation
Pre-operative management varies according to patient presentation. For the symptomatic patient seeking surgical intervention, a thorough pre-operative evaluation is recommended. Patients are generally older and often have significant co-morbidities requiring cardiopulmonary evaluation. Patients should undergo a barium esophagram [9] in order to define the anatomy, assess for volvulus, and evaluate for esophageal shortening, esophageal bolus transport and gastric emptying. Figure 13.2 shows a barium esophagram of a patient with a large type III para-esophageal hiatal hernia with all the stomach in the chest and with an organoaxial volvulus of the intrathoracic stomach. Upper endoscopy with biopsy is also routinely performed (preferably by the operating surgeon). Esophageal manometry can be attempted to assess esophageal motility to guide the surgeon’s operative planning with regard to the type of fundoplication and rule out esophageal motility disorders. High-resolution computed tomography, although not required pre-operatively, can depict the anatomical abnormalities well (Fig. 13.3).
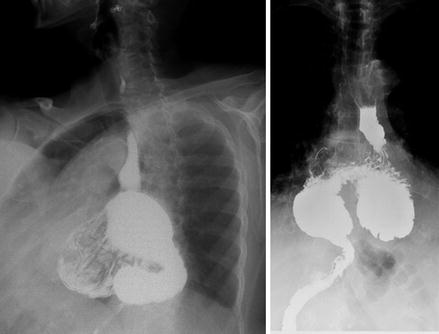
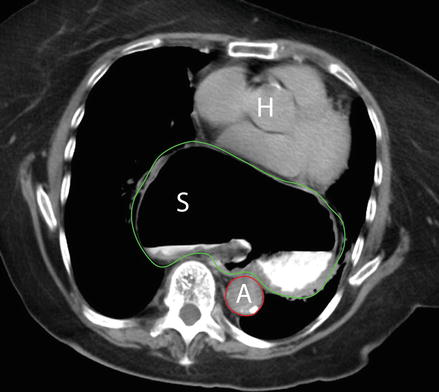

Figure 13.2
Barium esophagram showing large type III para-esophageal hiatal hernia with organoaxial volvulus of the intrathoracic stomach

Figure 13.3
Computed tomography of the chest showing a paraesophageal hernia. A large portion of the stomach S green outline, is noted high in the mediastinum, at the level of the heart H note the proximity of the posterior aspect of the hernia to the aorta (A red outline)
Positioning and Anesthesia
All patients should undergo induction of general anesthesia with endotracheal intubation using techniques to prevent aspiration. The patient should be placed in either the supine position or on a split-leg table. The arms are secured on padded arm boards at a 90° angle to the body’s axis. A footboard is placed and the patient secured to the bed in anticipation of a steep reverse Trendelenburg position during the case. An orogastric or nasogastric Levine tube can be placed as needed to decompress the stomach. Our preference is to introduce the Levine tube after the stomach is reduced and gastro-esophageal junction is under direct visualization. Sequential compression devices should be placed on the lower legs bilaterally and subcutaneous heparin can be administered for deep vein thrombosis prophylaxis. A foley catheter can be considered for close monitoring of urine output if the procedure is emergent or the patient has multiple comorbidities. The skin of the abdomen should be widely prepped after hair has been clipped short using an atraumatic electric clipper [10]. A single dose of antibiotics should be administered within 1 h of incision [11], typically a second-generation cephalosporin.
Operative Approach
Paraesophageal hernias can be repaired using laparoscopic, open trans-abdominal, or trans-thoracic (via a left thoracotomy) approaches. The laparoscopic approach is currently preferred in most centers. The possible benefits of the trans-thoracic approach include direct visualization of the sac and hernia, complete mobilization of the esophagus to the aortic arch and the ease of a relaxing incision on the left hemidiaphragm [3, 9]. Disadvantages include the pain of a thoracotomy, higher risk of peri-operative complications and the extended length of hospital stay [3, 9]. Most centers in the U.S. favor the use of laparoscopic techniques as complication rates seem lower than open approaches and recurrence rates with laparoscopic techniques seem similar to the ones obtained with the trans-thoracic and open abdominal approach [12, 13].
The laparoscopic approach to repair of paraesophageal hernias was first described in 1992 and emphasized crural approximation and fundoplication [14]. Five steps are essential components of a laparoscopic repair of a paraesophageal hernia:
One of the most important aspects of successful paraesophageal hernia repair is to create a tension-free repair. Axial tension can be addressed with mediastinal esophageal mobilization in most cases. Occasionally, a Collis gastroplasty may be necessary. Lateral tension, due to a large diaphragmatic defect, is usually addressed by using appropriate technique for crural closure or an absorbable synthetic or biologic mesh. However, in selected cases relaxing incisions with or without absorbable mesh placement may be beneficial [9, 12, 15]. Only absorbable synthetic or biologic mesh should be used at the hiatus. Non-absorbable meshes should be avoided as they have potential to erode into the esophagus. However, even the use of absorbable synthetic or biologic mesh is controversial since its efficacy in long-term objective recurrence has not been demonstrated [12]. Reduction of early recurrences with biologic mesh placement, however, may be one advantage over primary repair [16].
Laparoscopic repair is associated with less blood loss, fewer intraoperative complications, faster diet advancement, and shorter hospital stays [3, 17]. Unfortunately, the radiographic recurrence rate is still quite high, ranging from 23 to 50 % in selected series [15, 17–20]. However most radiographic recurrence are small recurrences of a small portion of the proximal stomach and the vast majority are asymptomatic, thus of little clinical significance and needing no treatment [18, 20].
Laparoscopic port sites are positioned in a configuration similar to that of a standard Nissen fundoplication. An additional 5 mm port in the left lower quadrant may be beneficial to assist with retraction and dissection. After inspection of the abdominal contents, the left lobe of the liver is retracted cephalad with a self-retaining laparoscopic retractor, exposing the hiatal defect and hernia (Fig. 13.4).
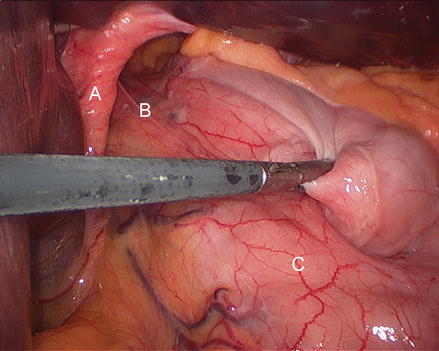

Figure 13.4
Reducing the stomach into the abdomen (A right crus, B preno-esophageal membrane, C stomach)
Description of the Laparoscopic Procedure
Reduction of Hernia Contents and Esophageal Hiatus Dissection
It is our preference to start the dissection at the gastrohepatic ligament, which is bluntly grasped and divided with the harmonic scalpel (Fig. 13.5). Care should be taken to identify and, if present, preserve a replaced left hepatic artery arising from the left gastric artery and traversing the gastrohepatic ligament. Next, the phrenoesophageal ligament is identified at the right crus. The ligament is grasped with an atraumatic grasper and pulled caudally. It is then divided with the harmonic scalpel, exposing an avascular plane while delivering the hiatal hernia sac medially (Fig. 13.6). Blunt dissection allows development of this avascular plane, which is extended cephalad into the thoracic cavity (Figs. 13.7 and 13.8). The dissection is then extended circumferentially in a clock-wise fashion to the left crus. Then, the peritoneal coverage of the right crus is opened and the avascular plane dissected to release the hernia sac in that location (Figs. 13.9, 13.10 and 13.11). Care is taken to identify and avoid entry into the pleura both the right and left side. Figure 13.12 shows the dissection to the base of the right crus and in proximity to the left gastric vessels. Dissection on the left side is facilitated by reduction of the gastric fundus (Fig. 13.13), opening the gastro-colic ligament, entering the lesser sac, and dividing the short gastric vessels (Figs. 13.14, 13.15, 13.16, 13.17, and 13.18). Some surgeons prefer to start the operation by identifying the left crus (as opposed to the right crus as described above) and then move to the right side. For this portion of the operation, we employ the harmonic scalpel. Clips should be placed on the larger and more proximal short gastric vessels (Fig. 13.17). Division of the short gastric vessels and mobilization of the stomach aids in complete circumferential dissection of the hernia sac (Figs. 13.19 and 13.20) and will facilitate the creation of a proper fundoplication, which is performed later in the operation.
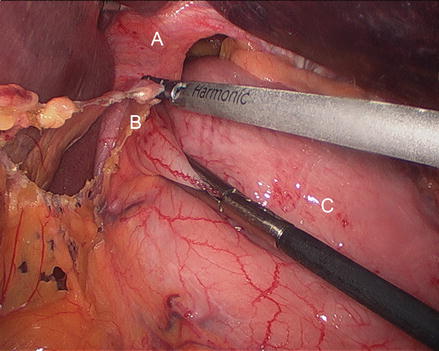
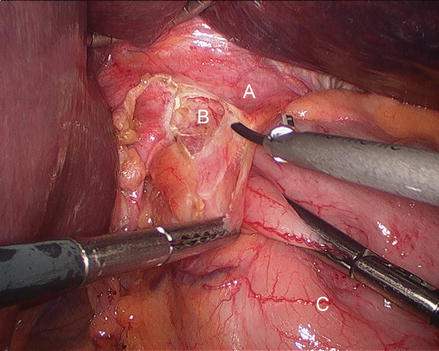
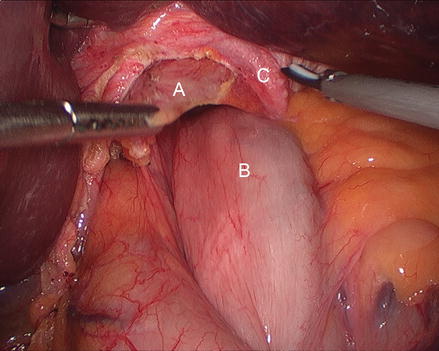
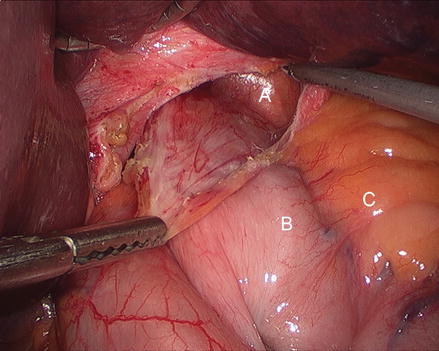
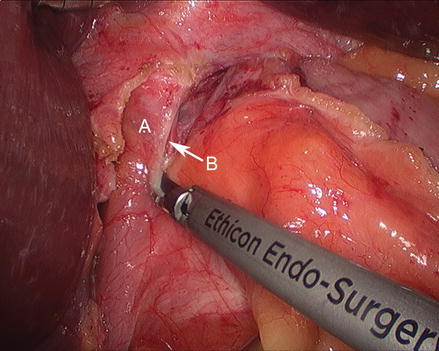
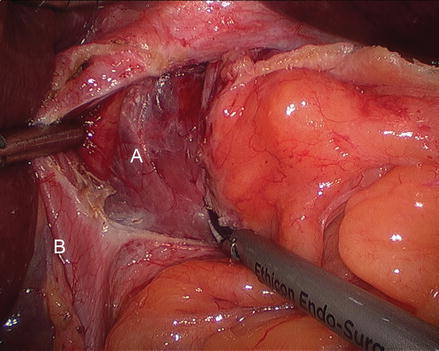
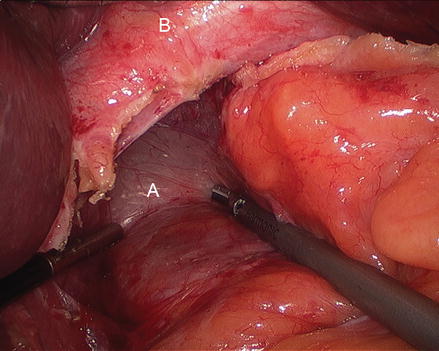
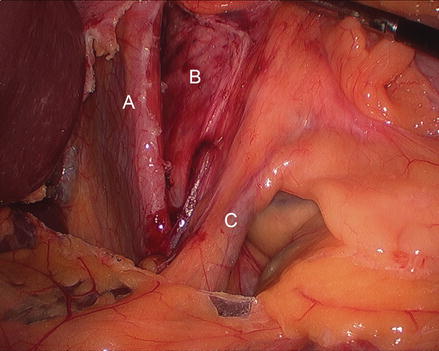
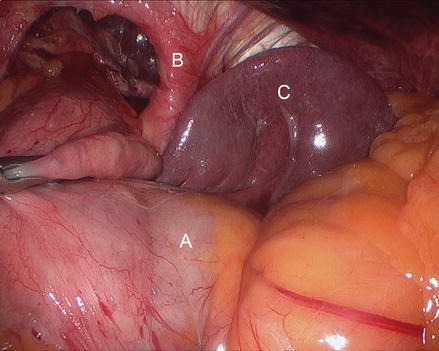
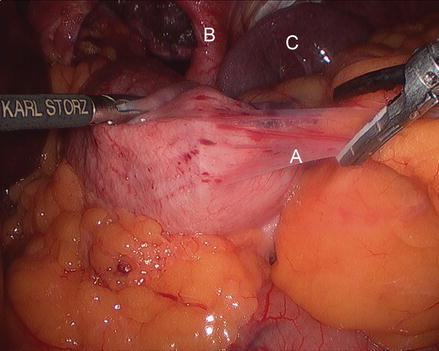
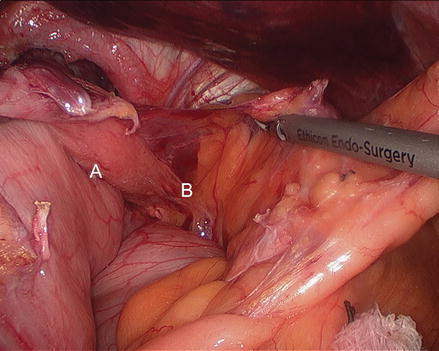

Figure 13.5
Division of the gastrohepatic ligament (A right crus, B gastrohepatic ligament, C stomach)

Figure 13.6
Division of the phrenoesophageal membrane (A phrenoesophageal membrane, B hernia sac, C stomach)

Figure 13.7
Dissection of the anterior aspect of the hernia sac (A hernia sac B gastric fundus C left crus)

Figure 13.8
Opening the hernia sac laterally (A areolar tissue inside hernia sac, B gastric fundus, C gastrosplenic ligament)

Figure 13.9
Incising the peritoneal coverage of the right crus (A right crus, B entering the avascular space to access the hernia sac)

Figure 13.10
Dissecting the avascular space in between right crus and the hernia sac (A avascular space, B right crus)

Figure 13.11
Dissecting the avascular space in between right crus and the hernia sac (A avascular space, B right crus)

Figure 13.12
Left gastric vessels (C) at the base of the hernia sac dissection (A right crus, B hernia sac)

Figure 13.13
Exposure of gastroesplenic ligament for dissection (A stomach, B left crus, C spleen)

Figure 13.14
Division of the short gastric vessels (SGV) for mobilization of the gastric fundus (A SGV, B left crus, C spleen)

Figure 13.15




Division of the short gastric vessels and mobilization of the stomach (A stomach, B SGV)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








