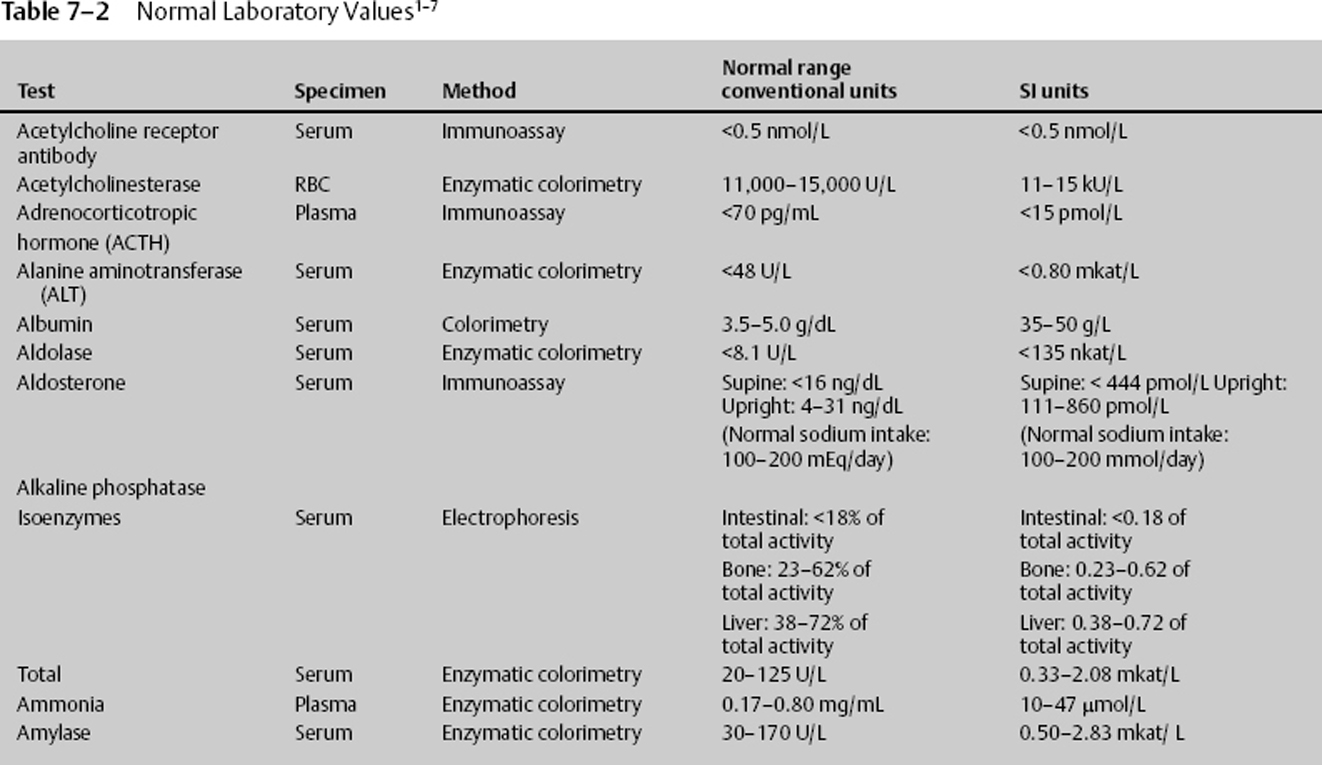
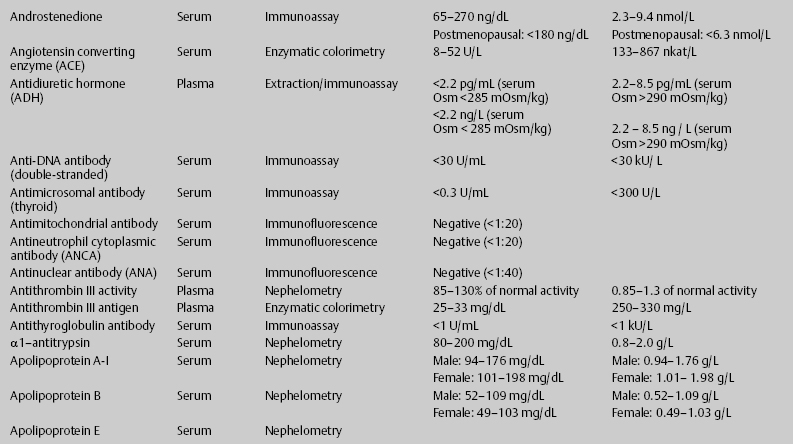
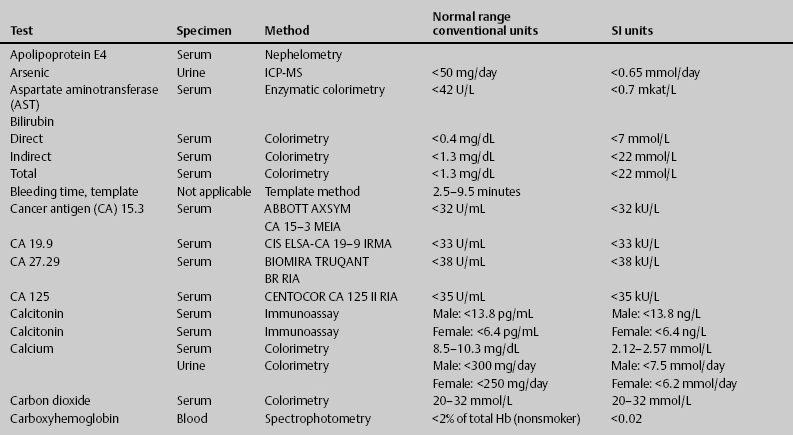
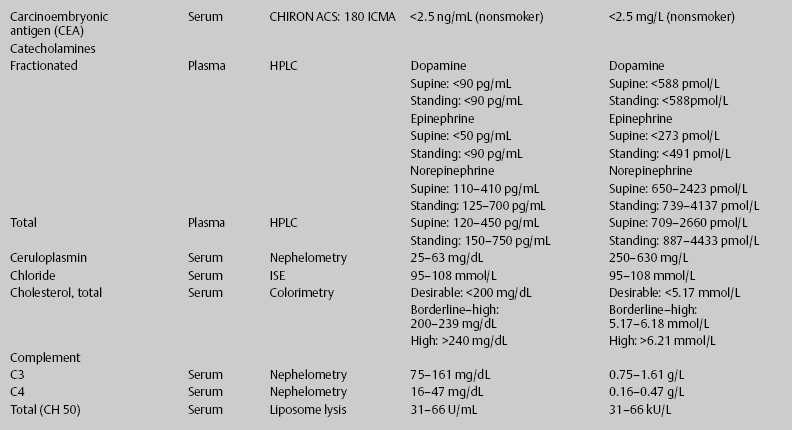
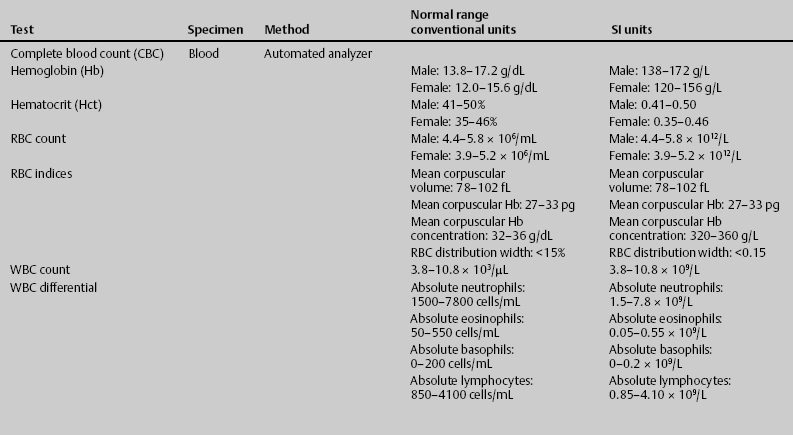
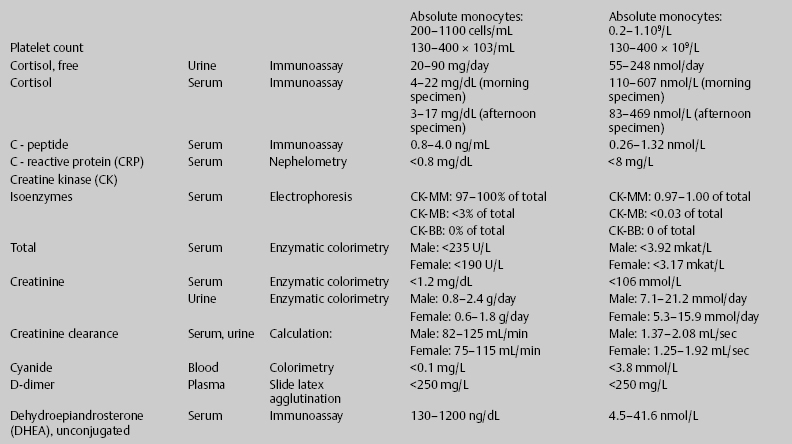
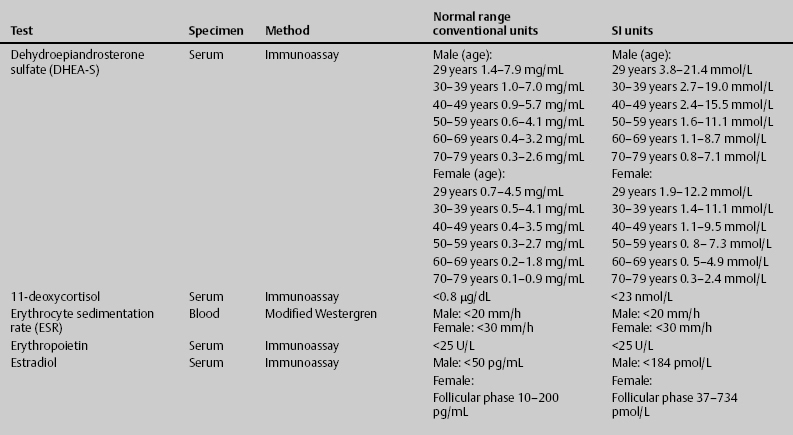
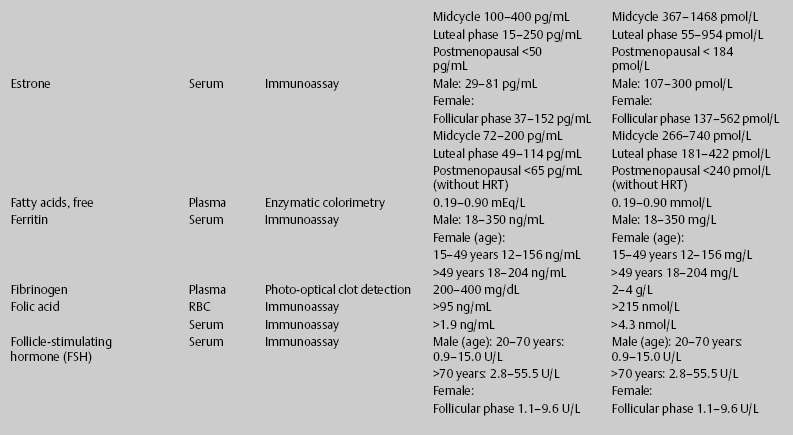
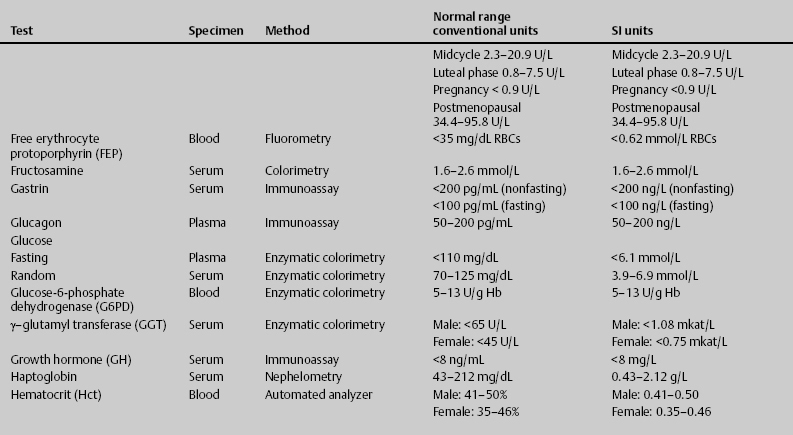
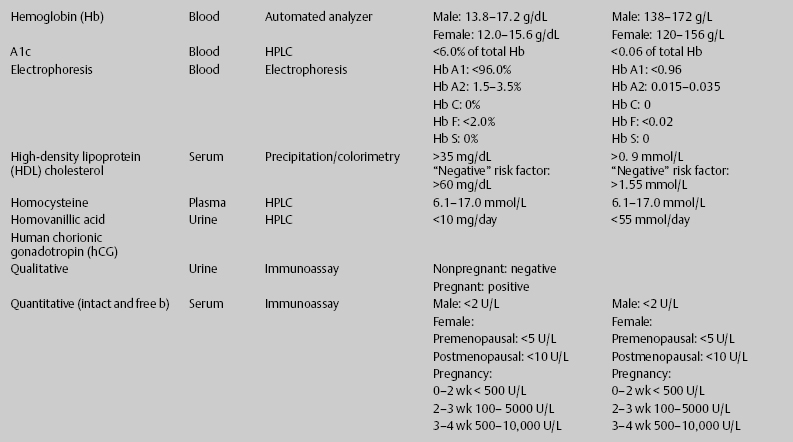
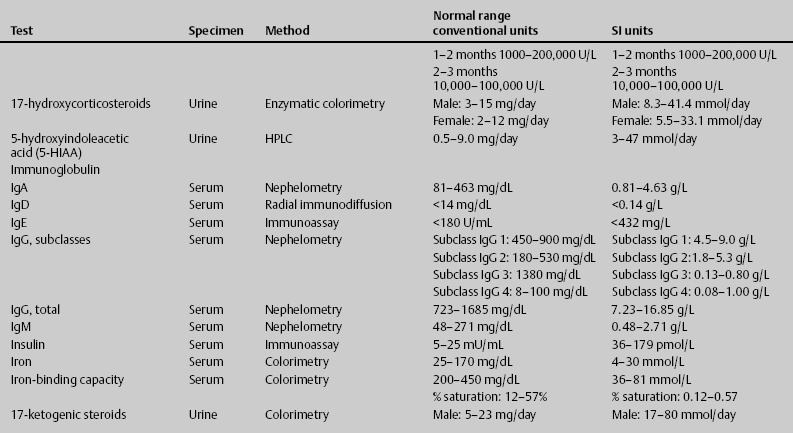
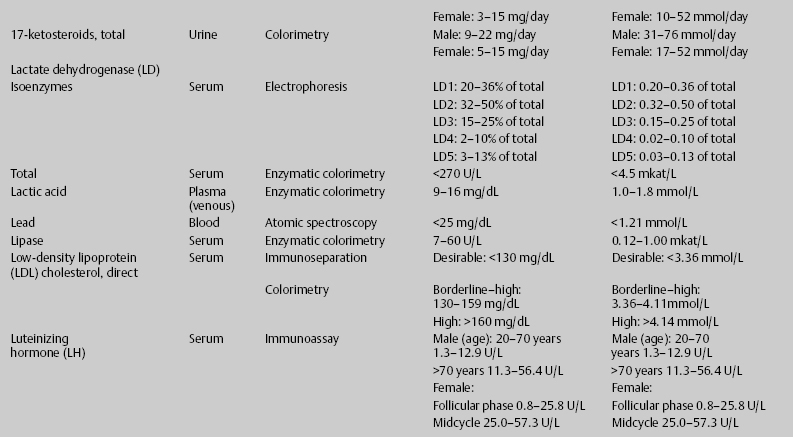
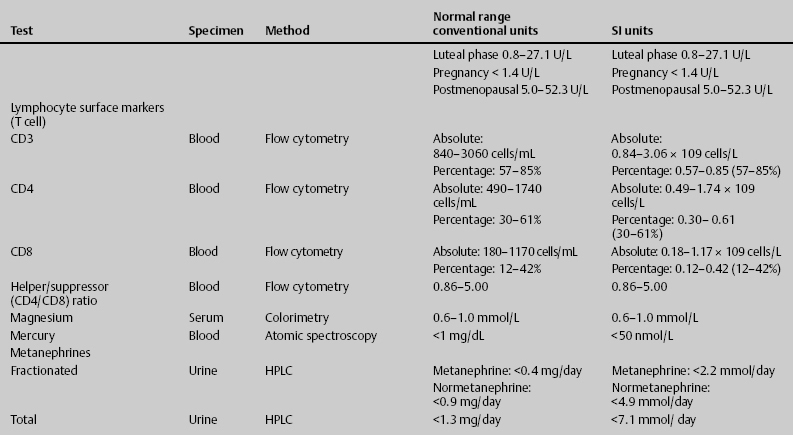
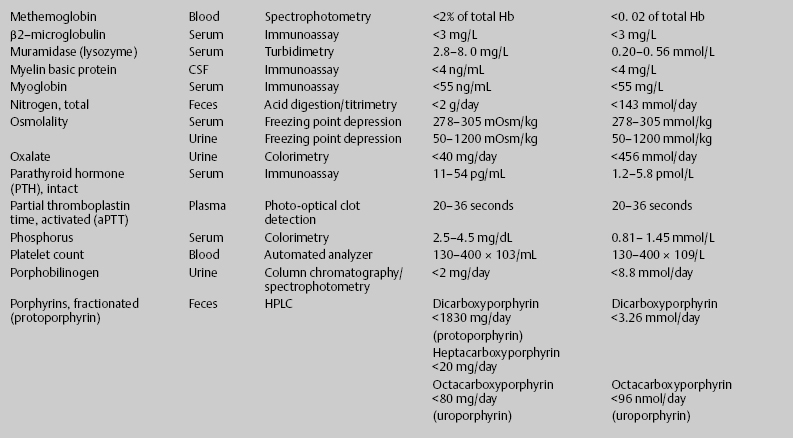
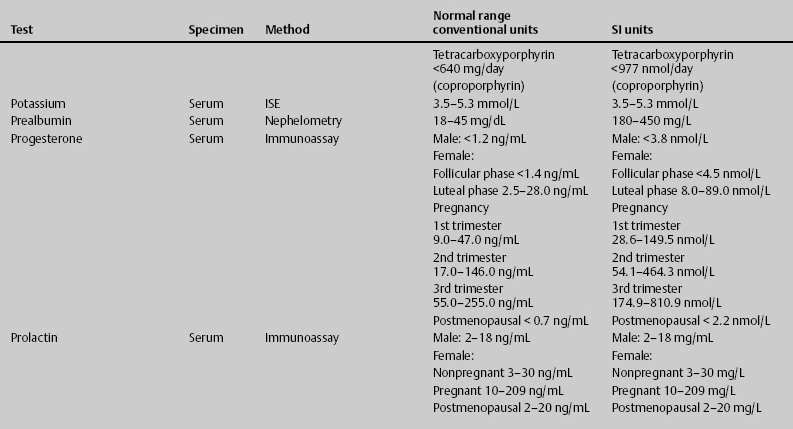
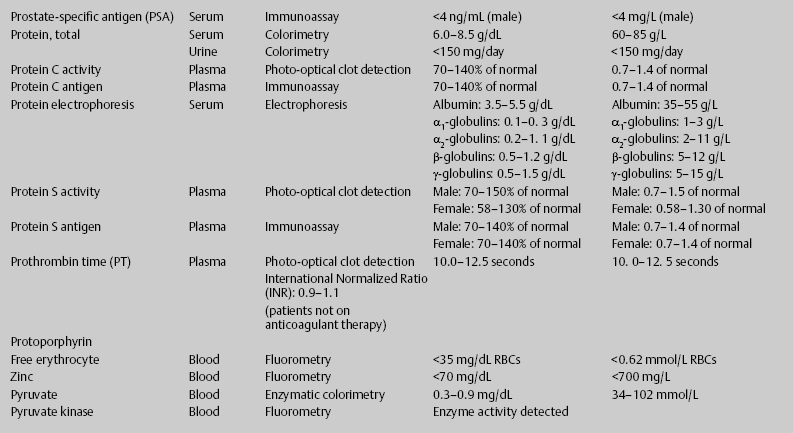
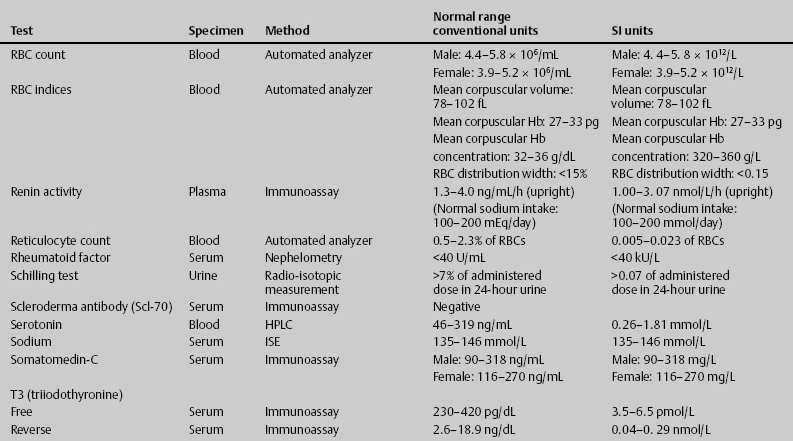
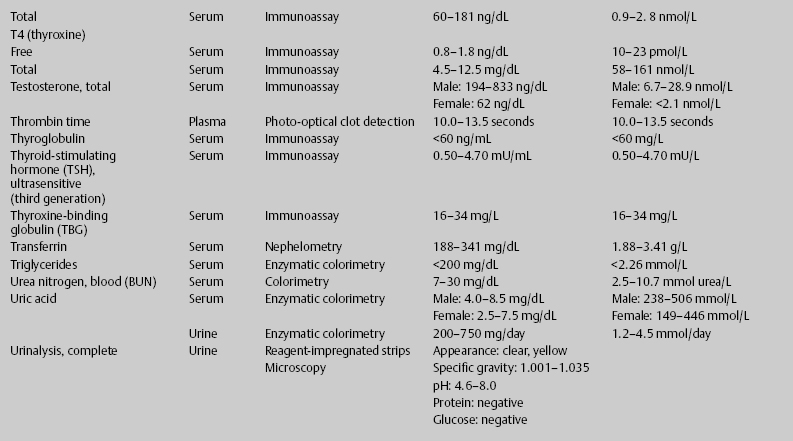
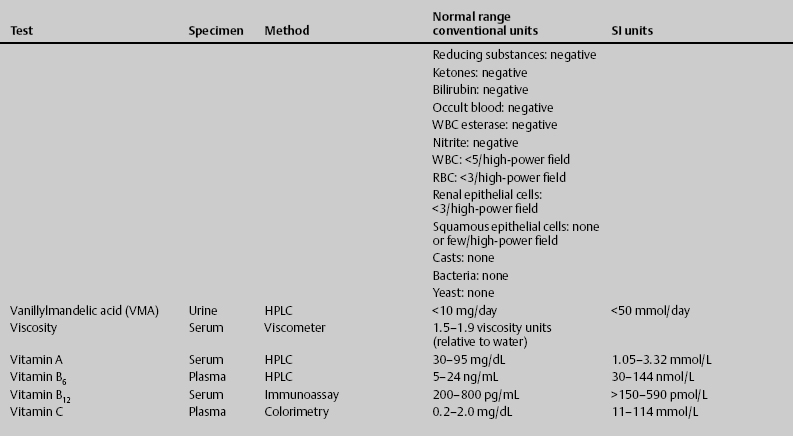
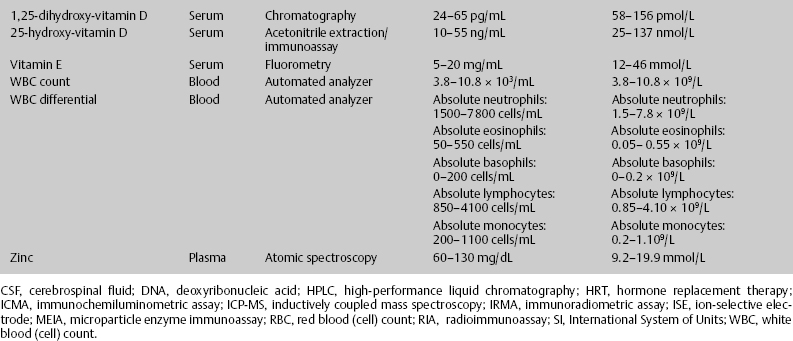
7 Jon Taveau Diagnostic laboratory studies are tools used to enhance the overall clinical picture. They assist in excluding or confirming a diagnosis when combined with thorough history and physical examination so that a treatment plan can ultimately be formulated and performed. Reassessing the patient and repeating diagnostic studies continue to confirm the diagnosis is accurate. This circular assessment sequence is the foundation of clinical medicine. It should be repeated as often as necessary. Ordering multiple studies indiscriminately, the so-called shotgun approach, adds much confusion to the assessment as well as a terrible cost burden. By using a strict sequenced clinical approach to diagnosis, costs, mistakes, and misdiagnoses are lessened. Tables 7-1 and 7-2 list common laboratory panels and normal laboratory values, respectively. The probability of a normal person having normal laboratory results when multiple tests are performed is relatively low. For example, one abnormal test result is expected in almost 50% of patients having a Chem-12 and 60% having a Chem-20. Chem-12 is a routine laboratory panel on serum that includes: sodium, potassium, chloride, CO2, creatinine, blood urea nitrogen [BUN], glucose, calcium, magnesium, phosphorus, albumin, total protein—also known as serum multiple analysis [SMA] 12. Chem-20 is also known as SMA 20. It is a routine panel of serum studies. Each lab determines the studies but typically it includes: albumin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, BUN, calcium chloride, CO2, creatinine, total and direct bilirubin, γ-glutamyl transterase, glucose, lactate dehydrogenase (LDH), phosphorus, potassium, sodium, protein, magnesium, and uric acid.)
Lab Work: What, When, and How Often?
 How Reliable Are Laboratory Results?
How Reliable Are Laboratory Results?
ACTH, adrenocorticotropic hormone; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogren; CBC, complete blood count; CK, creatine kinase; CK–MB, creatine kinase-MB mass; CMP, cytidine monophosphate; CSF, cerebrospinal fluid; DNA, deoxyribonucleic acid; ESR, erythrocyte sedimentation rate; FTA, fluorescent treponema absorption; HLA, human leukocyte antigen; Ig, immunoglobulin; INR, International Normalized Ratio; LDH, lactate dehydrogenase; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; PCR, polymerase chain reaction; RBC, red blood cell; TSH, thyroid-stimulating hormone; VDRL, venereal disease research laboratory; WBC, white blood cell.
Accuracy of laboratory values is also complicated by sex, age, diet, malnutrition, medications, time of day, and patient position. For example, plasma volume increases when the patient changes from upright to supine; age results in declining cardiac, pulmonary, renal, and metabolic functions; high serum potassium may be simply due to cellular release after prolonged tourniquet constriction or may be the result of hemolysis; low serum glucose may result in patients with high white blood cell (WBC) counts; or low serum sodium and high plasma hemoglobin may result in patients with hyperlipidemia.
Also, automated testing methods tend to be more subject to errors than manual methods, perhaps due to traces of sample remaining in instruments.1
 Preoperative Testing
Preoperative Testing
Routine preoperative laboratory tests should include a hemogram with differential (WBC, hemoglobin, hematocrit, and platelets), an electrolyte panel (sodium, potassium, chloride, carbon dioxide, BUN, creatinine, and serum glucose), and coagulation studies (prothrombin time [PT], International Normalized Ratio [INR], and partial thromboplastin time [PTT]). Depending on age and clinical suspicion, electrocardiogram (EKG) and chest x-ray may be appropriate. For all intracranial procedures and extensive spine procedures, blood should be typed and crossed for possible transfusion. For other neurosurgical procedures, blood should at least be typed and screened. Ancillary laboratory tests should be ordered based on the preoperative assessment and according to American Society of Anesthesiologists (ASA) guidelines.
The 2002 ASA Preoperative Testing Advisory1 is a loose set of guidelines that may be summarized as follows:
- No laboratory study may clear a patient for surgery. A physician must review the patient and state that “the patient is in optimal shape to undergo surgery.”
- Evaluation must occur prior to the day of surgery.
- Routine laboratory testing should be performed.
- Pregnancy testing should be routine for women of childbearing age (ages 11–55), irrespective of the patient’s belief that “there is no way” that she could be pregnant.
On the whole, not much benefit appears to result from nonindicated routine laboratory testing. There is little evidence to suggest that patients classified as ASA I or II benefit from laboratory testing. Furthermore, in the absence of laboratory tests in ASA I patients, medical care is not shown to be adversely affected.2
 Testing for Tumors
Testing for Tumors
Radiologic studies and surgical biopsy are the primary methods used to diagnose central nervous system (CNS) tumors; however, diagnostic laboratory studies may assist with diagnosis and therapeutic monitoring. Pituitary tumors may produce endocrine abnormalities that may be measured in serum. Polycythemia is associated with hemangioblastoma, a posterior fossa tumor. Some parasellar and pineal region embryonal tumors secrete hormones and proteins: α-fetoprotein (AFP), β subunit of human chorionic gonadotropin (β-hCG), and placental alkaline phosphatase (PLAP). Cerebrospinal fluid (CSF) cytology may be used to diagnose tumor type. CSF analysis may reveal polyamines (putrecine) that localize tumors to ventricles or subarachnoid space, or a pleocytosis that may increase suspicion for lymphoma.
Neuroepithelial Tumors
Astrocytomas, oligodendrogliomas, ependymomas, mixed gliomas, neuronal tumors, and other neoplastic processes of the CNS may be identified in CSF by cytology.
Pineal parenchymal tumors produce a characteristic clinical picture due to their proximity to the ventricular system. Opening pressure during lumbar puncture may be high due to obstructive hydrocephalus. Laboratory values reflecting endocrine dysfunction may be present in tumors invading the hypothalamus: diabetes insipidus, hypopituitarism, and precocious puberty. Biochemical tumor markers may suggest tumor type. Serum and CSF AFP, β-hCG, and PLAP levels are most commonly ordered to discriminate between choriocarcinoma or mixed germ cell tumors containing choriocarcinomatous elements (high β-hCG levels); germinomas, pineal parenchymal tumors, and teratomas (neither AFP nor β-hCG elevations); and germinomas (very high elevations of PLAP). However, tumor markers lack sufficient specificity and sensitivity to accurately predict tumor histology.
Meduloblastomas arise from the inferior medullary velum, then fill and expand into the fourth ventricle. High opening pressure during lumbar puncture may reflect obstructive hydrocephalus, and cytology from CSF analysis in 30% of patients will be positive for metastatic dissemination at the time of diagnosis (this is an indication of either drop metastasis or rostral dissemination supratentorially). Because meduloblastoma is characterized as the CNS neoplasm that most frequently metastasizes systemically (extraneural deposits occur in 5% of patients), diagnostic laboratory studies of bone and liver (alkaline phosphatase, γ-glutamyltransferase [GGT], and liver function test [LFT]) may result in vague, positive studies.3,4
Sellar Region Tumors
Pituitary tumors present clinically by symptoms of pituitary hypersecretion (70% of cases), symptoms of pituitary hyposecretion, or neurologic symptoms of compression of the pituitary or other adjacent sellar structures.
Hypersecretory syndromes include acromegaly, Cushing’s disease, amenorrhea-galactorrhea syndrome, and secondary hyperthyroidism from hypersecretion of growth hormone (GH), adrenocorticotropic hormone (ACTH), prolactin (PRL), and thyroid-stimulating hormone (TSH).
Hypopituitarism results in fatigue, weakness, hypogonadism, regression of secondary sexual characteristics, and hypothyroidism. Pituitary insufficiency also occurs acutely in pituitary apoplexy.
Chronic progressive compression of the pituitary is accompanied by decline in functional reserve of secretory elements: gonadotrophs are most vulnerable and are affected first, next thyrotrophs, somatotrophs, and finally corticotrophs (the most resilient among pituitary hormones). Acute processes produce the opposite effect. Panhypopituitarism results in compromise of all of the pituitary hormones, but hypocortisolism from ACTH deficit produces life-threatening adrenal insufficiency. Large adenomas may result in compression of the hypothalamus or in obstruction of CSF outflow and subsequent noncommunicating hydrocephalus. Also, inhibition of dopamine transport from the hypothalamus via the portal vessels to the anterior lobe of the pituitary results in loss of suppression of PRL output (the “stalk section effect”). This phenomenon may result in PRL levels elevating to 150 ng/mL or greater (normal levels are <25 ng/mL).
Diagnostic laboratory studies are very sensitive indicators of endocrine dysfunction and are focused on measuring pituitary and/or target-organ hormones in basal and provoked states to discriminate between various pituitary adenoma subtypes. Screening tests include (1) adrenal axis: morning serum cortisol, 24-hour urine free cortisol, dexamethasone suppression test, cosyntropin stimulation test, and insulin tolerance test; (2) thyroid axis: TSH, T4, and thyrotropin-releasing hormone (TRH) stimulation test; (3) gonadal axis: serum luteinizing hormone/follicle-stimulating hormone (LH/FSH); (4) prolactin levels: PRL; (5) growth hormone: somatomedin-C (insulin-like growth factor I [IGF-I]), growth hormone releasing hormone (GH-RH) stimulation test, and glucose suppression test; and (6) neurohypophysis: water deprivation test and serum antidiuretic hormone (ADH).
Craniopharyngiomas produce effects seen using endocrine studies. They may reflect arrested pituitary function in children (growth failure, diabetes insipidus, and primary amenorrhea) and some degree of hypopituitarism in adults. Also, opening pressures during lumbar puncture may be elevated due to mass-effect.3–5
Hematopoietic Tumors (Primary Central Nervous System Lymphoma)
Primary CNS lymphoma (PCNSL) is lymphoma of the CNS in the absence of systemic lymphoma. Systemic lymphoma disseminates to the meninges and rarely to the brain parenchyma, whereas primary CNS lymphoma frequently start as periventricular lesions.
PCNSL is rare and accounts for only 1 to 2% of intracranial tumors. It occurs primarily in the elderly; however, patients having human immunodeficiency virus (HIV) and congenital immunodeficiencies, collagen vascular disease, and organ transplants are particularly at risk for primary CNS lymphoma.
CSF cytology may detect high-grade disease and involvement of lep-tomeninges. The CSF analysis may reveal elevations in protein and pleocyto-sis (immunohistochemical stains reveal monoclonal populations of cells); β2-microglobulin, lactic acid dehydrogenase isoenzymes, and β-glucuronidase are elevated as well. Tissue biopsy is the preferred method of diagnosis of PCNSL. However, an unequivocally positive CSF cytology eliminates the need for brain biopsy.
Systemic lymphoma must be differentiated from PCNSL and is detected by analysis of the hemogram: polymorphonuclear leukocytosis may be present, lymphocytopenia occurs early and becomes pronounced with advanced disease, eosinophilia is present in ~20% of patients, and thrombocytosis and microcytic anemia develop with advanced disease. Also, elevated serum alkaline phosphatase levels indicate bone marrow or liver involvement, or both, and increases in leukocyte alkaline phosphatase, serum haptoglobin, erythrocyte sedimentation rate (ESR), serum copper, and other acute-phase reactants reflect active disease.3
Germ Cell Tumors
Germ cell tumors account for more than 50% of all pineal tumors. They range from low- or intermediate-grade to very aggressive high-grade tumors. For example, germinomas may metastasize within the spinal axis in 10% of patients and systemically in 3%, compared with aggressive pineal choriocarcinomas that disseminate throughout the craniospinal axis in 25% of cases and systemically in less than 5%. Cytology from CSF analysis may assist in diagnosis. However, highly aggressive germ cell tumors carry a grave prognosis and are frequently disseminated at the time of diagnosis.3
Nonmeningothelial Tumors of Uncertain Histogenesis (Hemangioblastomas)
Hemangioblastomas are benign neoplasms of uncertain origin. They are associated with a multisystem genetic disorder (Von Hippel-Lindau syndrome), pheochromocytomas, pancreatic and renal cysts, and renal cell carcinoma. Associated diagnostic laboratory studies may include complete blood count (CBC, for polycythemia from liberation of erythropoietin), measuring free catecholamines in a 24-hour urine specimen with a specific analysis for epinephrine (most sensitive) and vanillylmandelic acid excretion (often normal early on), LFT, amylase, lipase, creatinine clearance, and urinalysis (UA) (for hematuria), respectively.3
Local Extensions from Regional Tumors (Chordomas)
Chordomas may arise at any site along the axial skeleton. Preferred locations are the clivus (35%), sacrum (50%), and spine (15%). Regardless of location, all chordomas are locally invasive. They begin with destruction of bone at the site of origin, followed by infiltration of the dura, and eventually spread intracranially to encase vital cranial structures. Late dissemination (in 10–20% of cases) occurs to liver, lungs, heart, peritoneum, lymph nodes, and bone. Vague elevations in laboratory studies targeting these systems may be present, but only tissue biopsy is diagnostic.3
Metastatic Tumors
Metastasis to the brain and spinal cord is common among cancer patients. Brain metastasis occurs in 20% and spinal cord metastasis in 10% of patients having primary malignancies of the lung (bronchogenic carcinoma, especially small-cell carcinomas), breast cancer, renal cell carcinoma, melanoma, and gastrointestinal malignancies.
Primary lung cancers may present with several paraneoplastic syndromes, including carcinoid syndrome (symptoms caused by secretion of serotonin, prostaglandins, and other biologically active substances), Cushing’s syndrome (hyperadrenocorticism), hypercalcemia, and antidiuretic hormone secretion. Appropriate screening studies for paraneoplastic syndromes may assist in confirmation of disease but are not diagnostic. Sputum cytology can also be used to confirm the presence of central lesions but is not helpful for evaluation of peripheral solitary pulmonary nodules.
Renal cell carcinoma is usually silent; symptoms of hematuria, palpable flank mass, and flank pain denote advanced disease. Renal cell carcinomas secrete hormones (parathyroid hormone and erythropoietin) that cause hypercalcemia and erythrocytosis. Stauffer’s syndrome is a reversible hepatorenal condition with abnormal results on liver function studies.
Melanoma frequently spreads to lymphatics, liver, bone, brain, and lungs. In addition to comprehensive radiographic evaluation, serum alkaline phosphatase, γ-glutamyltransferase, and lactic dehydrogenase levels are ordered to evaluate for possible spread to bone and liver.3–5
 Cysts and Tumor-Like Lesions (Epidermoid and Dermoid Cysts)
Cysts and Tumor-Like Lesions (Epidermoid and Dermoid Cysts)
Epidermoid and dermoid cysts have a predilection for basal regions of the brain and tend to enlarge along CSF pathways. Common locations include the cerebellopontine angle, suprasellar region, fourth ventricle, and inside the diploë of the skull. Epidermoid tumors can cause local mass effect or recurrent bouts of aseptic meningitis due to leakage of irritative cyst contents (i.e., cholesterol and fatty acids) into the CSF. Lumbar puncture may result in high opening pressures as expected if hydrocephalus is present. CSF analysis may result in nonspecific leukocytosis. Alkaline phosphatase may be elevated due to destruction of bone.3
 Trauma
Trauma
Following trauma, several delayed conditions may present. Diagnostic laboratory studies may be implemented to diagnose and/or prevent delayed hemorrhage, post-traumatic diffuse cerebral edema, seizures, metabolic abnormalities, and meningitis.
Intracranial Hematoma
Initial or delayed hemorrhage may be anticipated in 75% of patients with traumatic brain injury. Epidural hematoma (EDH), subdural hematoma (SDH), or traumatic intracerebral hemorrhage (TICH) may be present in either initial or delayed fashions.
Following initial management (intubation, sedation, elevation of head of bed [HOB], and management of hypertension), the focus is turned toward correction of coagulopathy, administration of antiepileptic drugs (AEDs), and monitoring of electrolytes and osmolarity in anticipation of the onset of syndrome of inappropriate antidiuretic hormone (SIADH).
Coagulopathy is identified using PT, INR, PTT, bleeding time, and platelet count. Coagulopathy is treated with fresh frozen plasma (FFP) and vitamin K (phytonadione) and is carefully monitored using coagulation studies every 4 hours until it is corrected.
Phenytoin is the first-line antiseizure medication, and levels are observed as per the above protocol.
Serum and urine electrolytes, serum and urine osmolarity, and urine-specific gravity is ordered every 6 hours to screen for SIADH. It is important to record hourly fluid input and output. Serum uric acid may also be a helpful study to rule in the presence of SIADH (serum uric acid is decreased in SIADH).5–8
Diffuse Cerebral Edema
Increased cerebral blood volume may result from loss of cerebral vascular autoregulation. It occurs in children more frequently than in adults and when associated with severe head injury carries close to 100% rate of mortality.
Aggressive management of intracerebral pressure (ICP) is required. Along with general measures to lower ICP (elevation of HOB, sedation, normotension, and euvolemia), patients may be considered for hypertonic therapy with hypertonic saline or osmotic therapy using mannitol and Lasix. High tonicity (>320 mOsm/L) and hypovolemia (“running dry”) have little advantage to prevent cerebral edema and may result in renal dysfunction. Prevention of hypoglycemia is also important, as it aggravates cerebral edema.
Diagnostic studies should be geared toward monitoring of serum electrolytes and glucose, BUN, creatinine, and serum osmolarity. Also, hemoglobin and hematocrit should be measured to allow for optimization of volume status and oxygenation.5–7
Post-traumatic Seizure
Post-traumatic seizures may occur early (<7 days) or late (>7 days) following head trauma and may precipitate adverse events as the result of elevation of ICP, alterations in systolic blood pressure (SBP), changes in oxygenation, and excessive neurotransmitter release. Anticonvulsants may be used to prevent early post-traumatic seizures in patients that meet high-risk criteria: (1) presence of SDH, EDH, ICH, TICH, or delayed traumatic intracerebral hemorrhage (DTICH); (2) open-depressed skull fracture with parenchymal injury; (3) penetrating brain injury; or (4) seizure within the first 24 hours following trauma.
Patients meeting criteria should be started on phenytoin, carbamazepine, valproic acid, or phenobarbital for 1 week. Diagnostic laboratory studies to monitor AED levels are per specific agent:
- Phenytoin Peak serum drug concentrations are achieved between 3 and 12 hours after administration of an oral dose. Therapeutic drug concentrations can be obtained in 1 to 2 hours when the drug is administered intravenously. Time to steady state is highly variable, ranging from 7 to 10 days. Therapeutic range is 10 to 20 μg/mL
- Carbamazepine Single oral dose, the carbamazepine tablets and chew-able tablets yield peak plasma concentrations of unchanged carbamazepine within 4 to 24 hours. Carbamazepine suspension is absorbed faster than the tablet; peak plasma levels are reached within 2 hours. Time to steady state is 2 to 4 weeks. The therapeutic range is 6 to 12 μg/mL
- Valproic acid Peak serum drug concentrations are achieved between 1 and 4 hours with oral dosing. Time to steady state is between 2 and 4 days. Therapeutic range is 50 to 100 μg/mL
- Phenobarbital Peak serum drug concentrations are achieved between 1 and 6 hours after oral or intramuscular dosing. Time to steady state is 16 to 30 days. Therapeutic range is 15–30 μg/mL5–7
- Carbamazepine Single oral dose, the carbamazepine tablets and chew-able tablets yield peak plasma concentrations of unchanged carbamazepine within 4 to 24 hours. Carbamazepine suspension is absorbed faster than the tablet; peak plasma levels are reached within 2 hours. Time to steady state is 2 to 4 weeks. The therapeutic range is 6 to 12 μg/mL
Metabolic Disorders
Several metabolic disorders may result from trauma (directly or indirectly) that affect the CNS: hypoxia, hyponatremia, hypoglycemia, renal failure, and adrenal insufficiency, as well as hepatic encephalopathy.
Acute Renal Failure
Acute renal failure (ARF) is categorized as prerenal, postrenal, and intrinsic renal. Prerenal and postrenal causes are potentially reversible if diagnosed and treated early; some intrinsic renal causes that result in acute glomerular vascular and tubulointerstitial nephropathy are also treatable (malignant hypertension, glomerulonephritis, vasculitis, bacterial infections, electrolyte disorders [hypercalcemia], and drug reactions).
Prerenal azotemia (50–80% of ARF cases) results from inadequate renal perfusion caused by extracellular fluid volume depletion or cardiovascular disease. Postrenal azotemia (5–10% of ARF cases) results from various types of obstruction in the voiding and collecting systems. Intrinsic renal causes of ARF are the result of prolonged renal ischemia (hemorrhage, surgery) or a nephrotoxin.
Symptoms and signs relate to the loss of excretory function and depend on the degree of renal dysfunction, the rate of renal failure, and the cause (trauma, surgery). Preserved urine output of 1 to 2.4 L/day is common. Oliguria may occur; anuria suggests bilateral renal artery occlusion, obstructive uropathy, acute cortical necrosis, or rapidly progressive glomerulonephritis.
Prerenal azotemia may be suggested by any disorder lowering renal perfusion (excessive diuresis, dehydration, hemorrhage, pericardial tamponade, sepsis, liver failure, congestive heart failure [CHF], pulmonary embolism, transcellular fluid accumulation, ascites, peritonitis, pancreatitis, or burns). Postrenal azotemia should be sought in the absence of prerenal factors. Intrinsic renal disease causing acute tubular injury may have three phases. The prodromal phase varies in duration depending on causative factors (amount of toxin ingested, the duration and severity of hypotension). In prerenal azotemia, serum creatinine increases by 1 to 2 mg/dL/day (90–180 mmol/L) and BUN by 10 to 20 mg/dL (3.6–7.1 mmol urea/L). However, BUN levels by themselves may be misleading as an early index of renal function because they are elevated due to increased protein catabolism resulting from surgery, trauma, burns, transfusion reactions, or gastrointestinal or internal bleeding. Therefore, the ratio of BUN to creatinine must be known and should be no higher than 15 to 20:1. The oliguric phase lasts an average of 10 to 14 days, but it varies from 1 to 2 days to 6 to 8 weeks. Urine output varies between 50 and 400 mL/day. Nonoliguric patients have a lower mortality, morbidity, and need for dialysis. In the postoliguric phase, urine output gradually returns to normal; however, serum creatinine and urea levels may not fall for several more days. Tubular dysfunction may persist and is manifested by sodium wasting, polyuria unresponsive to vasopressin, or hyperchloremic metabolic acidosis.
The first step to diagnosing renal failure is to determine whether the renal failure is acute, chronic, or acute superimposed on chronic. Progression to chronic renal failure (CRF) is common when the serum creatinine concentration is >1.5 to 2 mg/dL This may occur even if the underlying disorder is not active. The definitive diagnostic tool is renal biopsy. Urea and creatinine levels are elevated. Plasma sodium concentrations may be normal or reduced. The serum potassium is normal or only moderately elevated (<6 mmol/L) unless potassium-sparing diuretics, angiotensin converting enzyme (ACE) inhibitors, p-blockers, or angiotensin receptor blockers are taken. Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), vitamin D metabolism, and renal osteodystrophy can occur; hypocalcemia and hyperphosphatemia are found regularly. Acidosis (plasma CO2 content, 15–20 mmol/L) and anemia occur. The anemia of CRF is normochromic-normocytic, with a hematocrit of 20 to 30%. It is usually caused by deficient erythropoietin production due to a reduction of functional renal mass. Urinary volume does not respond readily to variations in water intake. Urinary osmolarity is close to that of plasma (300–320 mOsm/kg). Urinalysis may reveal casts.5,6,9
Adrenal Insufficiency
Secondary adrenal insufficiency may occur in panhypopituitarism, in isolated failure of ACTH production, in patients receiving corticosteroids, or after acute discontinuance of longer use of corticosteroid therapy without appropriate tapering.
Panhypopituitarism occurs in a trauma setting resulting directly from destruction of pituitary tissue or secondarily from infection. Also, patients receiving corticosteroids for more than 4 weeks, or who have discontinued their use after a period of weeks to months, may have insufficient ACTH secretion during metabolic stress to stimulate the adrenals to produce adequate quantities of corticosteroids, or they may have atrophic adrenals that are unresponsive to ACTH.
Patients with secondary adrenal insufficiency are not hyperpigmented, as are those with Addison’s disease. They have relatively normal electrolyte levels. Hyperkalemia and elevated BUN are not present because of the near-normal secretion of aldosterone. Hyponatremia may occur on a dilutional basis. Patients with panhypopituitarism have depressed thyroid and gonadal function and hypoglycemia; coma may result when symptomatic secondary adrenal insufficiency occurs.
Function of the hypothalamic-pituitary-adrenal axis during long-term steroid treatment can be determined by the cosyntropin stimulation test: injection of 5 to 250 mg cosyntropin intravenously results in plasma cortisol level >20 mg/dL 30 minutes following the injection. Also, morning specimen cortisol, 24-hour urine free cortisol, dexamethasone suppression test, and insulin tolerance test may be used to test adrenal function.5,6,9
Hepatic Encephalopathy
Hepatic encephalopathy may result from hepatic failure resulting from trauma. The liver metabolizes and detoxifies digestive products brought from the intestine by the portal vein. In hepatic failure, these products escape into the systemic circulation if portal blood bypasses parenchymal cells or if the function of these cells is severely impaired. The resulting toxic effect on the brain produces the clinical syndrome.
Personality changes (inappropriate behavior, altered mood, impaired judgment) are common early manifestations. Psychomotor testing can detect such abnormalities not suspected clinically. Usually, impaired consciousness occurs. Initially, subtle sleep pattern changes or sluggish movement and speech may be present. Drowsiness, confusion, stupor, and frank coma indicate increasingly advanced encephalopathy. Constructional apraxia, in which the patient cannot reproduce simple designs (a star), is a characteristic early sign. A musty sweet odor of the breath, fetor hepaticus, occurs. A peculiar, characteristic flapping tremor due to lack of attention, called asterixis, is elicited when the patient holds his or her arms outstretched with wrists dorsiflexed; as coma progresses, this sign disappears, and hyperreflexia and the Babinski response may occur. Agitation or mania may occur. Seizures and localizing neurologic signs may also occur.
Diagnostic laboratory studies for hepatic function include serum total protein, albumin, alkaline phosphatase, ALT, AST, total bilirubin, ammonia (NH3), (GGT), prothrombin time, platelet count, and serum protein electrophoresis.
The diagnosis of hepatic encephalopathy is clinical. There is no correlation with liver function tests. Blood ammonia levels are elevated, but values correlate poorly with clinical status. The CSF is unremarkable except for mild protein elevation.
An electroencephalography (EEG) usually shows diffuse slow-wave activity, even in mild cases, and may be useful in questionable early encephalopathy.5,9
Post-traumatic Meningitis
Post-traumatic meningitis occurs in up to 20% of patients with moderate to severe head injuries. Most cases occur within 2 weeks of trauma; 75% of cases have demonstrable basal skull fracture, and 50% have obvious CSF rhinorrhea.
Antibiotic coverage for active infection should empirically be broad-spectrum, targeting gram-positive, gram-negative, and anaerobic organisms until culture and sensitivity results are known. They should be continued for at least 1 week following CSF sterilization.
Routine CSF analysis should include Gram’s stain, culture and sensitivity, protein, glucose, cell count with differential, color, and clarity. Pathogens frequently cultured following basal skull fracture include gram-positive cocci (Streptococcus haemolyticus, Staphylococcus warneri, Staphylococcus cohnii, Staphylococcus epidermidis, and Streptococcus pneumoniae), and gram-negative bacilli (E. coli, Klebsiella pneumoniae, and Acinetobacter ani-tratus). Elevations of protein and WBCs (polymorphonuclear neutrophil leukocytes), low glucose, and cloudy/turbid CSF suggest bacterial infection. CSF should be collected for CSF analysis weekly until sterilization is achieved.5–7
 Cerebrovascular Disease
Cerebrovascular Disease
Cerebrovascular Accident
Cerebrovascular accidents (CVAs) can be divided into ischemic and hemorrhagic. Approximately 80 to 85% of CVAs are ischemic, 15 to 20% are hemorrhagic.
Of patients presenting with focal deficit, 5% of cases are seizure, tumor, or psychogenic in etiology, and 95% vascular. Eighty to 85% of CVAs are attributed to ischemic infarct, whereas only 15 to 20% are from hemorrhage. With the mainstream use of thrombolytic therapy in the management of ischemic infarction, it is important to be familiar with the exclusion criteria used to make decisions regarding their administration. The use of anticoagulants (heparin or Coumadin), and extreme values of serum glucose may exclude a patient from receiving thrombolytic agents. Patients being considered for thrombolytic therapy should have coagulation studies as well as serum glucose screening prior to their administration.
Intracerebral hemorrhage (ICH) accounts for 15 to 20% of strokes. Etiologies of ICH include amyloid angiopathy, trauma, hemorrhagic transformation of an ischemic infarct, tumors, cerebrovascular malformations (cerebral aneurysms, arteriovenous malformations [AVMs], venous malformations, cavernomas, capillary telangiectasias). Several risk factors are associated with ICH: age, gender, race, prior CVA, alcohol consumption and substance abuse, cigarette smoking, and liver dysfunction. Diagnostic laboratory studies may be utilized in both diagnosis and management.
Factors complicating intracerebral hemorrhage are coagulopathy, seizure, and SIADH.
Coagulopathy is identified using PT, INR, PTT, bleeding time, and platelet count. Coagulopathy is treated with FFP and vitamin K (AquaMEPHYTON) and is carefully monitored using coagulation studies every 4 hours until it is corrected.
Phenytoin is the first-line antiseizure medication, and levels are observed as per the above protocol.
Serum and urine electrolytes, serum and urine osmolarity, and urine specific gravity are ordered every 6 hours to screen for SIADH. Serum uric acid may also be a helpful study to rule in the presence of SIADH (serum uric acid is decreased in SIADH).
Toxicology screening may be ordered if there is suspicion of substance abuse.5,6,8
Subarachnoid Hemorrhage
Many etiologies for subarachnoid hemorrhage (SAH) exist: rupture of intracranial aneurysms (80%), cerebral AVM (5%), spinal AVM, arterial dissection or rupture, vasculitides, tumors, coagulation disorders, dural sinus thrombosis, drugs, sickle cell disease, and pituitary apoplexy. Risk factors include hypertension, use of oral contraceptives, substance abuse, tobacco abuse, alcohol abuse, pregnancy, lumbar puncture, and advanced age. Along with radiologic studies, diagnostic laboratory studies may assist with diagnosis and are essential for management of SAH.
Patients having high suspicion for SAH will have noncontrast computed tomography (CT) performed. If CT is negative, lumbar puncture may be performed. Lumbar puncture is the most sensitive test for SAH, although false positives may occur as the result of a traumatic tap. Elevations of opening pressure and xanthochromia of CSF present during the lumbar puncture are consistent with SAH. CSF analysis will reveal nonclotting bloody fluid that does not clear with sequential tubes. Xanthochromia, or yellow discoloration, may take 48 hours to develop but may be seen early on. This may be subtle and will require fluid to be spun down to have supernatant evaluated via spectrophotometry (visual inspection is less accurate). Cell count reveals >100,000 RBCs/mm3, and when the first and last tubes are compared, there should not be a significant drop in counts. Protein elevations are from blood breakdown products. Glucose may be normal or reduced; RBCs present within CSF may metabolize glucose.
Once the diagnosis of SAH has been made, initial management is directed toward preventing rebleeding, detection and treatment of hydrocephalus and vasospasm, monitoring for hyponatremia, deep venous thrombosis (DVT) prophylaxis, seizure prevention, and determining a source of bleeding. Arterial blood gases (ABG), electrolytes, CBC, and PT/INR/PTT should be ordered on admission.
Coagulopathy is expeditiously corrected, and anticoagulants, including aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs), enoxaparin or other low-molecular weight heparin products, and Coumadin (warfarin) are discontinued. Therefore, DVT prophylaxis is by mechanical means: application of knee-high antiembolism (TED) hose and pneumatic compression boots. Coagulation panels should be monitored every 4 hours until coagulopathy is corrected, and then daily.
Blood rheology should be optimized using hemodilution; hemoglobin and hematocrit of 10 mg/dL and 30 to 35%, respectively, enhance perfusion when vasospasm is present.
SIADH and cerebral salt wasting may be detected by following serum and urine electrolytes, serum and urine osmolarity, and urine specific gravity.
AEDs are administered (phenytoin is preferred).5,6,8,10
 Brain Death
Brain Death
Laws Defining Criteria for Brain Death
Laws defining death existed at the state level, if at all, until medical technology advanced to require more strict criteria. A paradigm shift to establish universal guidelines for death, more specifically “brain death,” emerged surrounding successful use of live organs for transplantation. In 1981 the President’s Commission for the Study of Medical Ethical Problems11 addressed brain death and formed guidelines to establish a valid determination of death. The Uniform Determination of Death Act (1980) was created to help states further define, diagnose, and establish a protocol to collectively define brain death, although specifics were never made.
The Brain Death Examination
The brain death examination should be performed by a neurologist or neurosurgeon or, if unavailable, by a medical or surgical intensivist, anesthesiologist, or pediatrician.
The following procedures must be performed sequentially. First, a CT scan of the brain is ordered, then reviewed, and the injury is documented in great detail. Information describing large-size intracranial hemorrhages, massive ischemic strokes with brain shift, multiple hemorrhagic contusions, large extradural or subdural hematomas, diffuse brain edema with absent basal cisterns, fissures and sulci, and pontine hemorrhages with obstructive hydrocephalus are noted to explain the etiology of coma and loss of brainstem reflexes. In the event of a repeatedly normal CT scan examination, CSF could reveal diagnostic findings such as pleocytosis, increased erythrocyte count, or positive Gram’s stain. CSF should be collected for a polymerase chain reaction (PCR) specifically directed toward herpes simplex or rabies. Next, confounding factors must be ruled out; they must be excluded to proceed with a clinical examination to determine brain death.
The diagnosis of brain death cannot be made until hypothermia is excluded; until the core temperature has reached and been maintained above 32°C.
Acid–base abnormalities increase suspicion of intoxication. Respiratory acidosis is associated with opiates, ethanol, barbiturates, and anesthetics. Metabolic acidosis is common in acetaminophen, ethanol, and methanol, as well as ethylene glycol, salicylates, isoniazid, cyanide, cocaine, strychnine, and papaverine. Alcohol has a plasma half-life of 10 mL/hour, and the legal alcohol limit for determination of brain death is 800 to 1500 mg/L
Naloxone and flumazenil may be administered to patients suspected of opiate or benzodiazepine overdose. Patients are observed for at least 4 times the excretion half-life, assuming no half-life prolongation due to additional organ dysfunction, or (if suspicion of a drug intoxification remains high but cannot be identified) the patient should continue to be observed for a period of 2 days for a change in brainstem reflexes and motor response.
The presence of serum pentobarbital levels in patients with traumatic brain injury may be confusing when attempting to diagnose brain death. Barbiturates have been used in these patients to reduce increased intracranial pressure. Again, it is assumed that the clinical diagnosis of brain death can be made if the serum levels are less than the therapeutic range (less than 5 μg/mL).
Metabolic and endocrine irregularities can mimic brain death, for example, edema in hepatic failure and diabetic ketoacidosis. Many severe abnormalities such as hyponatremia, hypernatremia, hyperglycemia or hypoglycemia, hypothyroidism, panhypopituitarism, or Addison’s disease may decrease the level of consciousness and confound the neurologic examination.12
< div class='tao-gold-member'>