KEY POINTS
Jaundice (hyperbilirubinemia) is seen in critically ill patients and can occur due to prehepatic, intrahepatic, or posthepatic causes.
Biliary obstruction and acalculous cholecystitis are two common surgical problems requiring urgent intervention.
For acalculous or calculous cholecystitis, cholecystectomy removes the inflamed and ischemic gallbladder and prevents recurrence and thus is preferred in those able to tolerate the procedure. A cholecystostomy tube is indicated for nonsurgical candidates.
Diarrhea commonly occurs in critical illness (up to 60% of those on enteral feeds) and may be related to infection, medications, malabsorption, composition of the enteral feeds, or gastrointestinal disease.
Clostridium difficile should be ruled out as the cause of diarrhea in the ICU or any patient with risk factors (particularly antibiotics or contact) as morbidity and mortality increase with delay in treatment.
Fulminant Clostridium difficile can present as an ileus or with diarrhea in a toxic patient, and is associated with high mortality and frequent need for surgical intervention.
Studies are ongoing to determine the optimal medical and surgical management of Clostridium difficile. Currently for severe cases enteral vancomycin plus intravenous metronidazole is suggested ± subtotal colectomy or ileostomy with colon lavage.
Bowel obstruction should be ruled out prior to managing as pseudoobstruction.
Commonest causes of adult small bowel obstruction are adhesions and hernia, whereas commonest causes of adult large bowel obstruction are colon cancer, sigmoid volvulus, and stricture from diverticulitis.
Pseudoobstruction (nonmechanical obstruction) is managed by resuscitation, removing or limiting precipitants, using nasogastric or rectal tubes to relieve overdistension, and occasional endoscopic decompression or use of neostigmine in appropriate patients.
JAUNDICE
Jaundice is characterized by yellow discoloration of the skin, conjunctivae, and mucous membranes as a result of widespread tissue deposition of the pigmented metabolite bilirubin. It can present as an isolated abnormality, or associated with specific hepatic and/or pancreatic dysfunction, or associated with multisystem organ dysfunction.
In the intensive care setting, jaundice may be an important sign of a condition that requires ICU admission, such as acute cholangitis, or a new development in an already admitted patient, such as one with septic shock. Patient history, laboratory evaluation, appropriate imaging investigations, and a thorough understanding of those conditions that place a patient at increased risk for the development of hyperbilirubinemia will help narrow the broad differential diagnosis of jaundice and identify those conditions that require specific therapy.
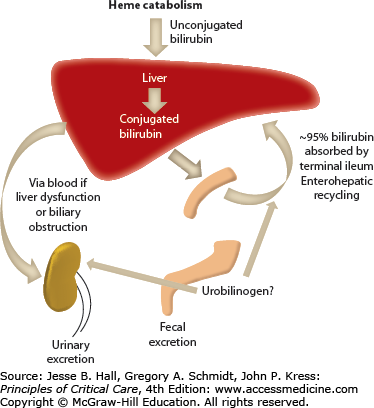
Bilirubin is a hydrophobic and potentially toxic compound that is an end product of heme degradation (Fig. 104-1 depicts bilirubin metabolism and excretion).1 The majority of bilirubin (70%-80%) is derived from degradation of hemoglobin from senescent erythrocytes, with a minor component of this being premature destruction of newly formed erythrocytes. The remaining 20% to 30% is mostly formed from breakdown of hemoproteins, such as catalase and cytochrome (CYP family) oxidases, in hepatocytes.
Bilirubin circulates in plasma tightly, but noncovalently, bound to albumin. To be excreted, bilirubin must be converted to water-soluble conjugates by hepatocytes and subsequently secreted in a multistep process. Bilirubin is taken up across the sinusoidal membrane of hepatocytes and conjugated with uridine diphosphate (UDP)-glucuronic acid by the enzyme bilirubin UDP-glucuronyl transferase (B-UGT). This converts the hydrophobic bilirubin into a water-soluble form that can be excreted into the bile canaliculus. Any conjugated bilirubin in plasma undergoes renal elimination and this pathway may be upregulated in disorders characterized by cholestasis. With prolonged cholestasis, a large proportion of conjugated bilirubin in plasma becomes covalently bound to albumin (referred to as delta bilirubin) which cannot be excreted into urine. Of note, this delta bilirubin will take longer to resolve than typical hyperbilirubinemias as its half-life becomes that of albumin, which is 14 to 21 days. Approximately 80% of bilirubin in bile is in the form of diglucuronides, with the rest being in the form of monoglucuronides and only trace amounts being unconjugated.
Normal serum bilirubin concentration in adults is less than 1.2 mg/dL or <20 µmol/L. Jaundice is generally not evident until serum concentrations exceed 3 mg/dL or 50 µmol/L. In healthy adults, <5% circulates in its unconjugated form.
Depending on the laboratory method of measurement, bilirubin concentration may be reported as total and conjugated, or potentially as total, direct, and indirect. Indirect bilirubin is not directly equivalent to unconjugated bilirubin and reliance on direct and indirect measurements can lead to errors in the diagnosis of isolated disorders of bilirubin metabolism. Measurement of the total and conjugated fraction is more useful. However, in disorders with prolonged cholestasis such assays may underestimate the conjugated bilirubin concentration because they do not accurately detect albumin-bound conjugated bilirubin (delta bilirubin). Even with modern assay techniques, the levels of total and conjugated bilirubin are often not able to distinguish hepatic disorders from biliary obstruction. Nevertheless, when combined with history and physical examination, jaundice can be characterized as obstructive or nonobstructive in over 75% of cases.
Table 104-1 lists possible causes of jaundice. The key step to determine management relies on differentiating whether the cause of hyperbilirubinemia is due to biliary obstruction or not.
Causes of Jaundice
| Prehepatic |
|
| Intrahepatic |
|
| Posthepatic |
|
Nonobstructive jaundice is often due to global hepatic or systemic disease for which the treatment is supportive and directed at the underlying disease. However, drugs and hepatotoxins as causative agents should be ruled out as specific time-sensitive antidotes exist (eg, n-acetyl-cysteine for acetaminophen toxicity).2
The common causes of obstructive jaundice in patients requiring ICU care include choledocholithiasis (stones in the common bile duct) with cholangitis,3 Mirizzi syndrome (cholecystitis with extrinsic compression of the common bile duct),4 biliary or pancreatic malignancies, severe pancreatitis, and postsurgical biliary strictures or complications. The importance in recognizing these causes is that many of them require surgical or urgent invasive therapies for effective treatment.
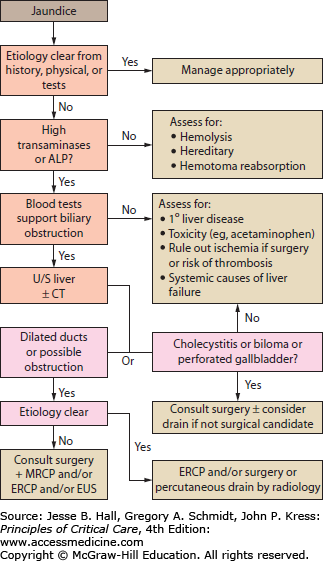
The patient’s history and physical examination provide important clues regarding the cause of jaundice. Important aspects of the history include presence of biliary and/or systemic symptoms, previous biliary tract disease or procedures such as ERCP, and previous biliary or intestinal surgery that may have resulted in altered biliary anatomy (eg, Whipple, Billroth II, bariatric procedures). The patient’s risk factors for viral infections should be assessed including any history of travel or blood transfusions. A history of alcohol use and/or abuse as well as exposure to hepatic toxins or recreational drugs is important. Any family history of hepatic or biliary disease, Gilbert syndrome, or hemoglobinopathy should be sought out. Physical examination may reveal abdominal scars, masses, areas of tenderness, or signs of existing liver disease. Laboratory investigation should begin with obtaining a CBC, alkaline phosphatase, serum transaminases (ALT and AST), bilirubin, lipase, albumin, and coagulation profile. Use of diagnostic imaging and modality will be guided by the clinician’s assessment of the most likely etiologies, but an abdominal ultrasound is a useful and common initial investigation. An algorithm for the investigation of jaundice is depicted in Figure 104-2.
Treatment of obstructive jaundice generally involves relief of the obstruction with invasive endoscopic, interventional radiologic, or surgical therapies. Nonobstructive causes largely require supportive therapy although specific medical therapies do exist for some diseases. Below we discuss several select causes of jaundice seen in the ICU setting.
Cholangitis: Acute cholangitis, also known as ascending cholangitis, is a bacterial infection of the biliary tract that occurs in an obstructed system and leads to systemic signs of infection.5 The leading cause of cholangitis is choledocholithiasis (common bile duct stones). The prevalence of gallstones (cholelithiasis) is estimated to be 10% to 20% in Western populations,6-8 with approximately one-quarter of these patients becoming symptomatic during their lifetime. Choledocholithiasis occurs in 10% to 20% of patients with symptomatic gallstone disease, and it’s these patients who are at risk for the development of acute cholangitis.5
The clinical diagnosis of acute cholangitis was first described by Charcot in 1877 and consists of the triad of fever, jaundice, and right upper quadrant pain. In 1959, Reynolds and Dargan added two other worrisome clinical findings (mental confusion and hypotension) associated with worse outcomes and this constellation of signs was subsequently termed Reynold pentad.9 The gold standard method of diagnosis is confirmation of biliary infection as the source of systemic illness by aspiration of purulent bile. However, this procedure is seldom done for diagnostic purposes, and thus the diagnosis of acute cholangitis continues to be made clinically. The diagnostic criteria developed by the Tokyo international consensus conference in 2006 are listed in Table 104-2.10
Diagnostic imaging in patients with cholangitis can serve to make the diagnosis as well as reveal the etiology of the biliary obstruction. Abdominal ultrasound, historically the first imaging test used in the investigation for biliary obstruction, continues to be extremely useful in the ICU setting. Its advantages include being able to be performed at a patient’s bedside, not requiring the use of intravenous nephrotoxic radiocontrast dye, being noninvasive, and widely available. Although obscuration of the distal common bile duct by overlying bowel gas is not uncommon and contributes to its lack of sensitivity for visualizing stones, it provides excellent visualization of the intrahepatic and proximal biliary tree and gallbladder, thereby allowing a diagnosis of biliary dilation to be made and the level of biliary obstruction (intrahepatic, proximal extrahepatic, or distal extrahepatic) to be determined. More recently, studies have demonstrated favorable accuracy of endoscopic ultrasound (EUS) and magnetic resonance cholangiopancreatography (MRCP) when compared to endoscopic retrograde cholangiopancreatography (ERCP),11 as well as favorable accuracy of computed tomographic cholangiography (CTC) when compared to EUS in the diagnosis of choledocholithiasis.12 EUS has been recently recommended by the American Society for Gastrointestinal Endoscopy as being highly accurate with fewer complications than ERCP in the detection of choledocholithiasis.13 Computed tomography provides imaging of not just the biliary system but the surrounding liver, pancreas, and foregut as well, making it an important investigative technique in determining the etiology of the biliary obstruction. ERCP is generally reserved as a primarily therapeutic procedure,3 although it may be used for diagnosis in centers without the availability of other noninvasive modalities.
Treatment of acute cholangitis depends on its severity and response to supportive therapies. Supportive care with early intravenous fluid resuscitation and broad-spectrum antibiotics is standard. The most common bacterial pathogens include Escherichia coli, Klebsiella, Enterobacter, Streptococcus, and Enterococcus,14,15 with Clostridium being the most common anaerobe. Patients with biliary stents in situ have a higher rate of polymicrobial infection (90% vs 45% of those without stents).15 Although no specific guidelines for antibiotic therapy exist, broad-spectrum coverage for the above listed common organisms including anaerobic coverage should be used, with definitive antimicrobial therapy based on the culture and sensitivity results obtained from blood if bacteremia is present and otherwise from bile cultures. However, the most important therapy is providing expeditious and effective biliary drainage. Without drainage the increased pressure in the biliary system creates ongoing cholangiovenous reflux of bacteria with resultant bacteremia and sepsis, as well as preventing effective secretion of antibiotics into the biliary system.14 A Cochrane review summarizes the superiority of ERCP compared to open surgery in the treatment of bile duct stones and cholangitis.16 Surgical and percutaneous biliary drainage is reserved for those cases where ERCP is unsuccessful or contraindicated such as some patients with a Roux-en-Y biliary-enteric anastomosis, bariatric procedures such as gastric bypass or duodenal switch, or a Billroth II reconstruction.17 Of note, removal of stones, if the causative etiology, is not necessary in the acute setting and can be performed electively at a later time so long as a stent can be successfully placed acutely.18 Patients admitted to the ICU with cholangitis are most likely to have a severe form and require early supportive therapy as outlined above as well as emergent biliary drainage.10,19 Patients without organ failure who respond to antibiotic therapy may be treated by ERCP within 24 to 48 hours.5
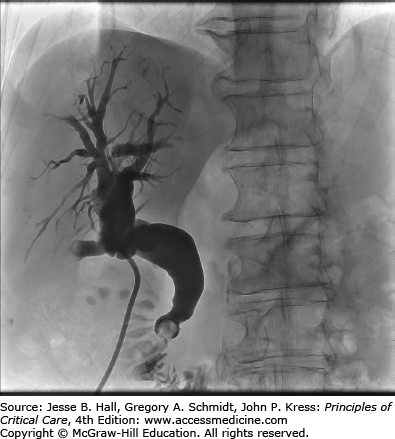
Patients who develop cholangitis as a complication of biliary stone disease should be referred for eventual elective cholecystectomy.19 These patients are at risk of recurrent cholangitis and other biliary complications (Fig. 104-3 illustrates biliary obstruction due to choledocholithiasis). A Cochrane review of over 600 patients randomized to endoscopic sphincterotomy or cholecystectomy for the treatment of choledocholithiasis demonstrated significantly reduced complication rates in the group that received an elective cholecystectomy as definitive treatment.20
Acalculous Cholecystitis: Acalculous cholecystitis is an acute inflammatory disease of the gallbladder that frequently presents in the ICU as fever or an elevated white count of unknown origin, or right upper quadrant pain.21 It is associated with elevated liver enzymes and jaundice in up to 20%. The pathophysiology is thought to be due to gallbladder stasis, endothelial injury, and ischemia leading to inflammation and necrosis of the gallbladder. A number of infections (eg, Epstein-Barr, cytomegalovirus, Campylobacter jejuni, Vibrio cholera) are also associated with development of acalculous cholecystitis, along with more commonly seen ICU risk factors such as prolonged lack of enteral feeds, total parenteral nutrition, mechanical ventilation, burns, shock, sepsis, massive transfusion, diabetes, renal failure, and cardiovascular disease.
Ultrasound is the investigation of choice, although the diagnosis can also be made by CT. Management consists of appropriate resuscitation, broad-spectrum intravenous antibiotics (covering enteric bacteria such as E Coli, Enterococcus, Klebsiella, Pseudomonas, Proteus, and Bacteroides), and prompt surgery consultation. For those able to tolerate the operative procedure without undue risk, laparoscopic cholecystectomy provides definitive source control and prevents recurrence. In those deemed not an appropriate candidate for surgery, a percutaneous drain placed by interventional radiology has been shown to be almost as effective as surgery in most patients.22 Providing the patient recovers, studies have shown that subsequent cholecystectomy is not needed in all patients.23 In such cases, the percutaneous drain is left in place for several weeks to ensure development of a fibrous tract, and a cholangiogram is done via the tube to ensure the absence of persistent gallbladder or biliary obstruction or leak, prior to drain removal.
Morbidity and mortality of acalculous cholecystitis increases with delay in diagnosis and management, with mortality as high as 75% having been reported in critically ill patients. As such, a high index of suspicion and early diagnosis plus surgery consultation are recommended.
Parenteral Nutrition–Associated Cholestasis: Total parenteral nutrition (TPN) is associated with a number of significant side effects including steatosis, lipidosis, and cholestasis.24 The mechanisms are multifactorial, with TPN promoting bacterial overgrowth in enterally unstimulated bowel, which in turn favors conditions known to induce cholestasis such as translocation of intestinal endotoxins into the portal venous system, bacterial sepsis, and formation of lithogenic bile acids.25 Long-term TPN therapy results in gallbladder akinesis, biliary stasis, and biliary sludge that promotes the formation of gallstones, which in turn contribute to obstructive forms of jaundice as well as acalculous cholecystitis. Persistent parenteral nutrition–associated cholestasis (PNAC) can progress to cirrhosis and eventual liver failure.25 Efforts to treat PNAC include cyclical TPN, decreasing dextrose and fat amounts, promoting enteral nutrition, treating bacterial overgrowth, and discontinuation of TPN altogether.25
Postsurgical, Trauma, and Other: Jaundice is a common postoperative complication of surgery, most commonly occurring in cardiac surgery,26 hepatobiliary surgery including liver transplantation, and surgery complicated by, or for the treatment, of sepsis.27 Not unexpectedly, hepatobiliary and pancreatic surgeries are associated with multiple potential complications (vascular thrombosis, strictures, leaks, hepatic insufficiency), and thus in such patients, early surgical consultation is advised.28,29
Bile leakage into the peritoneal cavity may be reabsorbed by the peritoneal lining and manifest as hyperbilirubinemia, such as in a post-laparoscopic cholecystectomy missed bile duct injury, perforated acalculous cholecystitis, or severe liver trauma. Another interesting but rare cause of jaundice in postoperative or trauma patients is hematobilia which often presents as jaundice, severe right upper quadrant pain, and melena. Hemolysis can also occur in the postoperative setting especially in patients who received large amounts of red blood cell transfusions, those with hemoglobinopathies including sickle cell disease, and those susceptible to any pro-oxidant medications (eg, patients with glucose-6-phosphate dehydrogenase deficiency) that may have been given perioperatively.
Severe hypotension and ischemia can also produce a condition termed “shock liver”30 with a clinical pattern of a rapid rise in serum aminotransferases to levels 10 to 100 times the upper limit of normal, along with delayed and less significant rises in bilirubin. Levels subsequently plateau within a few days and the fall steadily with return to normal levels.24 Gilbert syndrome, characterized by a relative deficiency of hepatic UDP-glucuronyl transferase, may also become unmasked by the stress of surgery or infection and a self-limited asymptomatic rise in bilirubin may occur. There are also many drugs that can cause injury to the liver and an examination of the patient’s medications and doses, including any herbal or recreational agents used, is important when searching for an etiology of jaundice.
DIARRHEA
The occurrence of gastrointestinal complications in the critically ill patient is common.31 A multicenter study of 400 patients conducted in Spain in 1999 found that diarrhea complicated 15% of patients admitted to the ICU.32 A similar study of over 1300 ICU patients published 10 years later reported a 14% incidence of diarrhea.33 The occurrence of diarrhea continues to complicate the care of ICU patients and its management is an ongoing challenge, especially in the face of recommendations for earlier and more aggressive enteral feeding.34,35 This section will discuss the approach and management to diarrhea that develops in the critical care patient, followed by a separate discussion of Clostridium difficile.
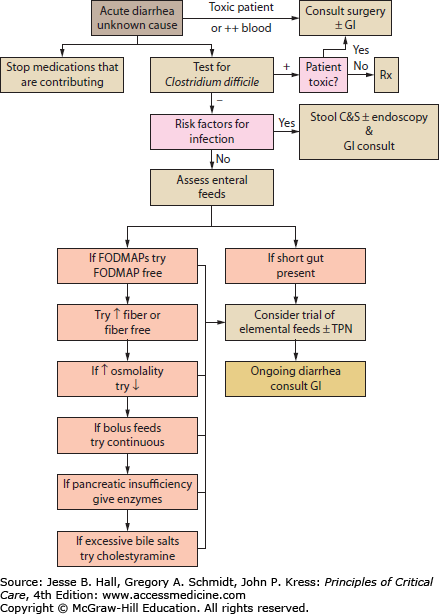
While diarrhea can be classified into osmotic, secretory, infectious, or noninfectious, the causes of diarrhea in the critically ill patient can be simplified to those due to infection, medication, oral or enteral feeds, or preexisting intestinal disorders of absorption or motility. Most diarrhea in the ICU is acute in onset (<14 days) as opposed to persistent ≥14 days or chronic ≥4 weeks.36 Infection must always be considered and ruled out in any new-onset diarrhea (see Fig. 104-4 and Tables 104-3 and 104-4). A careful history including any collateral information from the patient’s family or close associates may reveal a preexisting condition such as lactose intolerance, celiac disease, inflammatory bowel disease, or irritable bowel syndrome. Investigation generally involves fecal specimen analysis for common bacterial and viral pathogens, and assessment for the presence of fecal toxins when infection with C difficile or enterotoxigenic/enterohemorrhagic bacteria is suspected. Routine testing for ova and parasites is not cost-effective for the majority of patients,37 but should be considered in the setting of persistent diarrhea, patients with a history of travel to a high risk area, and immunocompromised patients.36
Common Causes of Acute Infectious Diarrhea in Developed Countries
| Bacteria | Salmonella, Campylobacter, Shigella, E coli 0157, Clostridium difficile |
| Viruses | Caliciviruses (eg, norovirus), rotavirus, adenovirus, astrovirus |
| Protozoa | Cryptosporidium, Giardia, Cyclospora, Entamoeba histolytica |
Major Causes of Chronic Diarrhea
| Osmotic |
|
| Fatty |
|
| Inflammatory |
|
| Secretory |
|
Medications are another cause of diarrhea by a variety of mechanisms. Antibiotics are frequently associated with diarrhea by altering the colonic flora, and laxatives and prokinetics increase intestinal motility. Acid-suppressive medications also have an inherent propensity to cause diarrhea (up to 7% of proton-pump inhibitors) and many oral electrolyte formulations or antacids are known irritants to the gastrointestinal mucosa (magnesium, phosphates). A study of 27 ICU patients treated for constipation showed 70% of them subsequently developed diarrhea,38 and another study showed diarrhea resolved in over 25% of patients following the discontinuation of laxative therapy.39 Many oral medications are hyperosmolar and/or contain sorbitol which can cause GI intolerance especially when given in large volumes. Sorbitol is a sugar alcohol that is used as a sweetener in many oral liquid medications and is known to cause osmotic diarrhea and cramping when ingested in amounts over 10 to 20 g in healthy volunteers.40 The amount of sorbitol is often not specified on medication labels as it is an inactive ingredient, and thus the amount of sorbitol being delivered to a patient is often difficult to determine.
Fluid and electrolyte repletion is an important initial therapy as large-volume diarrhea can quickly lead to significant fluid, electrolyte, and acid-base disturbances; repletion should be accomplished via the intravenous route until the etiology of the diarrhea is determined.
The patient’s medication list should be examined for causative agents and these should be discontinued or substituted with alternative medications or routes of administration when appropriate. Sorbitol-containing liquid medications should be discontinued and sorbitol-free formulations or crushed tablets used when available. Dilution of any necessary hyperosmolar medications should be considered.41,42
Antimotility and antidiarrheal agents should be reserved for those patients whose diarrhea persists despite the identification and treatment of the underlying cause. C difficile infection should specifically be ruled out as antimotility agents in this setting can precipitate the development of a toxic megacolon.43 Antimotility agents include loperamide, diphenoxylate/atropine, and oral narcotic derivatives. Loperamide is advocated as the medication of choice as it has the lowest risk of central nervous system adverse effects.42 Bismuth subsalicylate is less effective than loperamide and lacks supporting data to recommend its use.44 Cholestyramine is effective in the treatment of diarrhea caused by bile acid malabsorption (eg, patients with short bowel syndrome, terminal ileum resection, postcholecystectomy) but given concerns about binding to other medications—most notably oral vancomycin45—and the lack of data outside of these specific patient populations, its general application is not recommended.
The composition of enteral nutrition formulas (ENF) can also be responsible for diarrhea and modification of these components can bring resolution.31 ENF with high osmolality may cause diarrhea, especially when being fed directly into the small bowel, and changing to a lower osmolality formula may alleviate diarrhea.46,47 Some ENF may contain poorly absorbed and rapidly fermentable short-chain carbohydrates collectively termed FODMAPs (fermentable, oligo-, di-, monosaccharides, and polyols).41,48 These act similarly to undigested lactose and include fructooligosaccharides (FOS), galactooligosaccharides (GOS), and fructose. FODMAPs significantly increase output from the small bowel due to osmotic effects and present rapidly fermentable substrates to colonic bacteria with subsequent excessive and ongoing gas production. Dietary FODMAPs have even been shown to induce symptoms in healthy volunteers,49,50 and their role in intestinal dysmotility in the ICU is an area of active research.
Fiber is also often incorporated into the ENF or be added as a supplement. Fiber can be classified as soluble or insoluble, and each has different effects. Soluble fibers, typically found in fruits and vegetables51 (eg, partially hydrolyzed guar gum, fructooligosaccharides, pectin, inulin, psyllium), are fermented by colonic anaerobic bacteria to short-chain fatty acids.42 These fatty acids are a preferred fuel for colonocytes and may mitigate diarrhea by improving sodium and water reabsorption in the colon.52 Insoluble fibers, typically found in whole grains (eg, cellulose and hemicellulose) may decrease diarrhea by increasing stool bulk and absorbing water.42,51 Many types of fiber have been studied in the prevention and treatment of diarrhea in patients. Conflicting meta-analyses have been published53,54 although the more recent analysis including over 1700 patients in 51 studies showed a reduction in the incidence of diarrhea with fiber supplementation of enteral feeds.53 The SCCM and ASPEN guidelines state that the use of soluble (but not insoluble fiber) may be useful in patients who develop diarrhea while receiving enteral nutrition.34
Modification of the microflora of the gastrointestinal tract is another area of active investigation and its role in the ICU has yet to be determined. Probiotics are a preparation or product containing viable defined microorganisms in sufficient numbers which alter the microflora by implantation or colonization in a compartment of the host and that exert beneficial effects in the host. Prebiotics are nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth or activity of one or a limited number of bacteria in the colon, and thus improve the health of the host. Synbiotics are a combination of prebiotics and probiotics able to modulate gut immunity and facilitate nutrient/factor interaction necessary for gut recovery.55 A review of the literature by Isakow in 2007 found no evidence for the use of probiotics in critically ill patients.56 The SCCM/ASPEN Clinical Practice Guidelines published in 2009 state there exist insufficient data to make recommendations for general usage in the ICU population.34 There are currently a number of active trials investigating their use in the ICU population.
Finally, the method of delivering enteral feeds can also cause diarrhea with continuous feeding potentially having a lower incidence compared with intermittent feeding.57,58 Bacterial contamination of enteral feeds has also been postulated as a cause of diarrhea although data for this are lacking.34
The Clostridium difficile bacillus was first described in 1935, although its association with disease was not identified until 1978.59Clostridium difficile infection (CDI) has become an increasingly common cause of health care–associated diarrhea and an increasingly common reason for admission to the intensive care unit (ICU).60-63
The prevalence of asymptomatic colonization with C difficile is 7% to 26% among adult inpatients in acute care facilities.43 The primary reservoirs of C difficile
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree