Patients presenting for CEA and carotid angioplasty and stenting (CAS) procedures are often elderly, have advanced cerebrovascular disease, and frequently have significant coexisting diseases involving other organ systems.
 Anesthetic management of these patients requires both an understanding of the physiologic stress imposed by the surgical procedure (disruption of the major cerebral hemispheric blood supply) and an appreciation of the physiologic constraints imposed by coexisting diseases.
Anesthetic management of these patients requires both an understanding of the physiologic stress imposed by the surgical procedure (disruption of the major cerebral hemispheric blood supply) and an appreciation of the physiologic constraints imposed by coexisting diseases.
 Current guidelines for CEA are based on large multicenter prospective studies that confirm the efficacy of CEA as a surgical procedure for the prevention of stroke in appropriately selected patients.
Current guidelines for CEA are based on large multicenter prospective studies that confirm the efficacy of CEA as a surgical procedure for the prevention of stroke in appropriately selected patients.
 Numerous studies emphasize the important role that patient selection, preoperative optimization of comorbidities, and the experience and the expertise of the surgeon, surgical team, and institution play in mitigating risk associated with CEA.
Numerous studies emphasize the important role that patient selection, preoperative optimization of comorbidities, and the experience and the expertise of the surgeon, surgical team, and institution play in mitigating risk associated with CEA.
 A large body of evidence supports the premise that, in experienced centers, the procedure can be performed safely under regional or general anesthesia.
A large body of evidence supports the premise that, in experienced centers, the procedure can be performed safely under regional or general anesthesia.
 Although use is not linked to evidence of improved outcome, many centers employ intraoperative neurologic monitoring to guide the management of patients who develop cerebral ischemia in response to carotid occlusion.
Although use is not linked to evidence of improved outcome, many centers employ intraoperative neurologic monitoring to guide the management of patients who develop cerebral ischemia in response to carotid occlusion.
 Stroke and myocardial infarction remain the most prominent causes of postoperative morbidity and mortality emphasizing the importance of appropriate patient selection and preoperative optimization.
Stroke and myocardial infarction remain the most prominent causes of postoperative morbidity and mortality emphasizing the importance of appropriate patient selection and preoperative optimization.
 CAS procedures are associated with a higher risk of stroke and death compared to CEA and current recommendations do not support the use of these procedures in asymptomatic patients or routinely in symptomatic patients. CEA remains the “gold standard” surgical procedure for the prevention of stroke.
CAS procedures are associated with a higher risk of stroke and death compared to CEA and current recommendations do not support the use of these procedures in asymptomatic patients or routinely in symptomatic patients. CEA remains the “gold standard” surgical procedure for the prevention of stroke.
I. GUIDELINES FOR PERFORMING CEA
A. Overwhelming evidence over the past two decades supports the efficacy of CEA, combined with best medical therapy, for the prevention of stroke among appropriately selected, medically stable patients who have symptomatic carotid stenosis (Table 11.1).
B. Current American Heart Association (AHA) guidelines1,2 recommend CEA for patients who have symptomatic carotid stenosis of 50% to 99% if the perioperative risk of stroke or death is < 6% and for asymptomatic patients who have stenosis of 60% to 99% if the risk of perioperative stroke or death is < 3% (Table 11.2). These recommendations are based on clinical trials that incorporated stringent inclusion criteria and note has been made that the risk associated with CEA is also influenced by clinical conditions that bear on outcome, such as life expectancy, gender, comorbidity, and surgical experience.
TABLE 11.1 Number of Patients Needed to Treat with CEA (NNT) to Prevent Death or Stroke
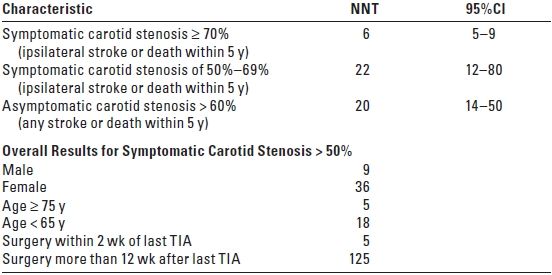
CEA, carotid endarterectomy; NNT, number needed to treat; CI, confidence intervals; TIA, transient ischemic attack. Adapted from Rothwell PM, Eliasziw M, Gutnikov SA, et al. Lancet. 2003;361:107–116 and Lancet. 2004;363:915–924. Based on post-hoc analysis of 5-year pooled data from major CEA trials assuming a risk of stroke or death following CEA of 6% or less for symptomatic patients and 3% or less for asymptomatic patients.
TABLE 11.2 Candidates for Carotid Endarterectomy
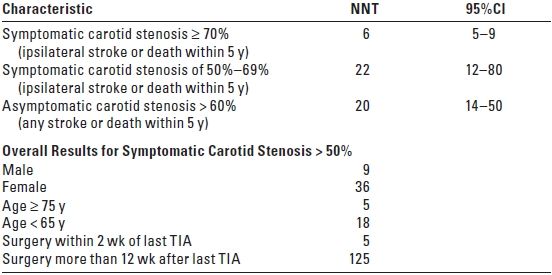
TIA, transient ischemic attack. Adapted from Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Carotid Endarterectomy—an evidence-based review. Neurology. 2005;65:794–801.
C. Subgroup analyses of the pooled results of the major North American and European CEA trials report that CEA is of greatest benefit for elderly males (≥ 75 years) with a high-grade (≥ 70%) symptomatic stenosis who proceed to surgery within 2 weeks following the last ischemic event (TIA).3
D. Endovascular treatment for carotid stenosis—CAS—has advanced markedly over the past two decades. Endovascular approaches to carotid revascularization are currently widely employed around the world. However, despite numerous clinical trials, definitive evidence demonstrating superior outcome relative to CEA has not been forthcoming.4
E. CEA remains the preferred surgical intervention for the prevention of stroke for patients who have extracranial cerebrovascular disease.
II. PHYSIOLOGIC CONSIDERATIONS
A. Cerebral blood flow (CBF) and metabolism
1. The brain is highly active metabolically but is essentially devoid of oxygen and glucose reserves, making it dependent on the continuous delivery of oxygen and glucose by the cerebral circulation.
2. CBF is provided by the internal carotid arteries (approximately 80%) and the vertebral arteries (approximately 20%), which anastomose at the base of the brain to form the circle of Willis.
3. Patients who have advanced occlusive cerebrovascular disease may be dependent on other collateral channels to maintain adequate CBF.
4. Normally, CBF is autoregulated to match the brain’s metabolic requirements and maintain normal neuronal function.
B. Cerebral perfusion
1. CBF is related directly to cerebral perfusion pressure (CPP) and inversely to cerebrovascular resistance (CVR).
a. CPP is defined as the difference between mean arterial blood pressure (MAP) and intracranial pressure or central venous pressure, whichever is higher.
b. CVR is related to blood viscosity and the diameter of the cerebral resistance vessels.
2. Optimization of CBF during CEA is managed indirectly through the factors most amenable to intraoperative manipulation—arterial blood pressure and arterial carbon dioxide tension (PaCO2), which impact CPP and CVR, respectively.
C. Carbon dioxide tension PaCO2
1. Within the range of PaCO2 from 20 to 80 mm Hg, CBF changes by 1 to 2 mL/100 g/minute for every 1 mm Hg change in PaCO2.
2. Coexisting disease processes such as diabetes mellitus and severe chronic obstructive lung disease may impair the cerebrovascular response to carbon dioxide.
3. In the absence of demonstrable benefit associated with hypercarbia or hypocarbia during CEA, the most common approach to ventilatory management during these procedures is to maintain normocapnia. This is achieved by ventilation to a PaCO2 that produces a normal pH in the absence of coexisting metabolic acidosis.
D. Blood pressure
1. Normally, CBF remains stable within the range of MAP from 50 to 150 mm Hg. Beyond this range, the limit of vasomotor activity is exceeded and CBF directly depends on changes in CPP.
2. In patients who have chronic hypertension, both the upper and the lower limits of autoregulation are shifted to higher pressures.
3. In patients who have cerebrovascular disease, the CBF response to changes in PaCO2 during carotid cross-clamping is impaired. Under these conditions, improvement in CBF is likely to depend largely on increases in CPP, emphasizing the relatively greater importance of blood pressure control during CEA surgery.
4. During CEA, blood pressure should be maintained within the normal preoperative range. Mild increases in systolic blood pressure of up to 20% above normal at the time of cross-clamping are acceptable, but hypotension and severe hypertension should be avoided.
III. PREANESTHETIC ASSESSMENT
A. The patient’s state of health is determined from the medical history, pertinent physical examination, and chart review.
B. Coexisting diseases are assessed and optimized. Common coexisting diseases include coronary artery disease, arterial hypertension, peripheral vascular disease, chronic obstructive pulmonary disease, diabetes mellitus, and renal insufficiency.
C. For patients who have diabetes mellitus, perioperative blood glucose should be carefully managed to avoid both hypo- and hyperglycemia. Current evidence suggests that perioperative hyperglycemia (bloodglucoses level > 200 mg/dL) adversely affects outcome following CEA.5 Hypoglycemia is also neurologically deleterious. As a consequence, perioperative glucose management should probably be targeted at glucose levels that are consistent with the AHA guidelines for the management of acute stroke (140 to 185 mg/dL).6
D. Cardiac complications remain a prominent source of mortality after CEA. The benefit associated with CEA is premised on evidence that the risk of stroke in the absence of treatment is higher than the risk of stroke or death as a consequence of treatment. Minimizing perioperative risk by optimizing cardiovascular comorbidities (e.g., hypertension, coronary artery disease, and congestive heart failure) represents a key determinant of outcome.
E. Cerebral angiograms, including computed tomographic or magnetic resonance angiography studies, should be reviewed to identify patients at increased risk from the presence of significant contralateral carotid artery disease or poor collateral circulation.
F. A risk stratification scheme for perioperative complications has been proposed for patients undergoing CEA (Table 11.3). Recently, several other risk indices for CEA have been developed and variably validated. Key common features suggest that active coronary artery disease, a history of congestive heart failure, significant contralateral carotid disease (particularly occlusion), and unstable neurologic ischemic symptoms merit particular caution in patients presenting for CEA.
TABLE 11.3 Preoperative Risk Stratification for Patients Undergoing CEA
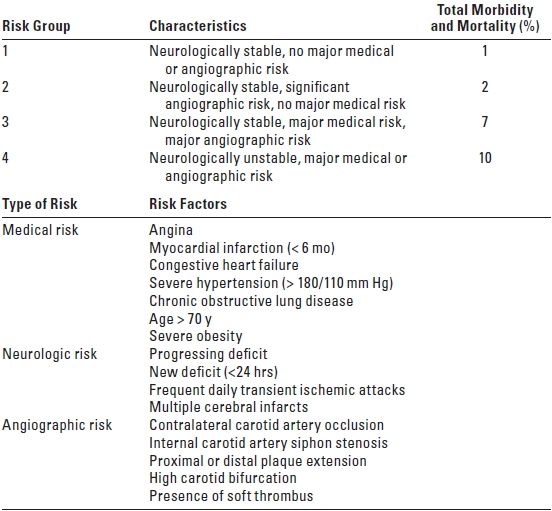
Adapted from Sundt TM Jr, Sandok BA, Whisnant JP. Carotid endarterectomy. Complications and preoperative assessment of risk. Mayo Clinic Proc. 1975;50:301–306. Used from Herrick IA, Gelb AW. Occlusive cerebrovascular disease: anesthetic considerations. In: Cottrell JE, Young WL, eds. Anesthesia and Neurosurgery. 5th ed. St Louis: Mosby, 2010:282, with permission.
IV. ANESTHETIC MANAGEMENT
CEA can be safely performed under general anesthesia, regional anesthesia, or local anesthetic infiltration. Experienced centers report similar morbidity and mortality. Despite enormous interest, available evidence, including the results from a recent large prospective trial (GALA), subgroup analyses of data from the prospective CEA trials, and meta-analyses of the published literature, remains insufficient to establish the definitive superiority of any one technique.7,8 As a consequence, it is likely that outcome following CEA is more likely to be favorably influenced by patient selection, optimization of concurrent disease, and meticulous surgical technique than by the choice of anesthetic.
A. Regional anesthesia
1. Superficial or deep cervical plexus blocks are the most commonly used regional anesthetic techniques for CEA.
a. A superficial cervical plexus block is performed by injecting a local anesthetic subcutaneously along the posterior border of the sternocleidomastoid muscle where the cutaneous branches of the plexus fan out to innervate the skin of the lateral neck.
b. A deep cervical plexus block is a paravertebral block of the C2–C4 nerve roots. This technique involves injecting local anesthetic at the vertebral foramina (transverse processes) of the C2–C4 vertebrae to block the neck muscles, fascia, and greater occipital nerve.
c. The two blocks are reported to be equally effective for CEA. However, deep cervical plexus block has been reported to have a higher failure rate (inadequate block) and to represent the exclusive source of serious, although rare, complications associated with these blocks. As a consequence, superficial cervical plexus block has been advocated as the regional technique of choice for CEA.9
d. Many regional anesthesia textbooks describe these techniques in detail and should be reviewed before performing the blocks.
e. With experience and patience, CEA can also be performed satisfactorily under local anesthetic infiltration of the surgical field and underlying tissue planes.
2. Intraoperative monitors include intra-arterial blood pressure measurement, continuous electrocardiogram (ECG), pulse oximetry, and capnography—often sampled via nasal prongs to monitor respiratory rate when the patient’s head and neck may not be entirely visible under the surgical drapes.
3. Supplemental oxygen should be provided through a mask or nasal prongs positioned to avoid the site of surgery.
4. Carefully titrated sedation using small, repeated, intravenous doses of fentanyl, 10 to 25 mcg, and/or midazolam, 0.5 to 2 mg, should render the patient comfortable and cooperative during the operation. Propofol is a reasonable alternative administered typically as a low-dose continuous infusion at 10 to 75 mcg/kg/minute and can be supplemented with a low-dose infusion of remifentanil (0.04 to 0.08 mcg/kg/minute).
5. The alpha 2-agonists, dexmedetomidine and clonidine, have also been reported to provide sedation and modest analgesia with minimal respiratory depression.10 Limited experience with these drugs during CEA suggests they are associated with reduced intraoperative hypertension but a higher incidence of postoperative hypotension. Careful attention is necessary during the administration of dexmedetomidine to avoid hemodynamic instability (i.e., transient hypertension, hypotension, and bradycardia).
6. Equipment should be immediately available to convert to general anesthesia if intraoperative conditions warrant.
7. Advantages of regional anesthesia include the following:
a. Superior neurologic monitoring associated with an awake patient
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






