Some isotopes, such as tritium, are unstable and undergo radioactive decay. This is a random process that follows an exponential pattern; the half-life of an isotope is the time taken for its mass to halve. Rates of decay are measured in Becquerels (Bq).

The curie was traditionally used to measure decay, which is based on the activity seen in 1 gram of radium-226, which equals 3.7 × 1010 Bq.
Decay emits radiation, which can be of three types.
α-radiation: a helium nucleus (two protons and neutrons) is emitted.
β-radiation: a neutron splits into a proton and an electron, emitting the electron at high velocity. Occasionally a positively charged electron is emitted, known as a positron.
γ-radiation: electromagnetic waves of wavelength <10−12 m. They occur whenever α– or β– radiation is emitted.
These types of radiation are classed as ionising, as they are capable of displacing electrons from around the nucleus of atoms they collide with to form ions. Ionising radiation is potentially damaging to body cells and DNA. The SI unit for absorbed dose is the gray (Gy), defined as 1 joule of energy deposited in 1 kilogram of mass.
The type of radiation and the tissues being irradiated influence the clinical outcome. Alpha radiation is 20 times more ionising than gamma radiation, and rapidly dividing tissues (such as ovarian tissue) are more susceptible to ionising effects. For this reason, the sievert (Sv) is a more useful clinical measure of radiation exposure; it is the amount of radiation equivalent in biological effectiveness to one gray of gamma radiation. The sievert is generally used for low levels of radiation absorption, and is tissue-specific.
X-rays are waves of electromagnetic radiation, similar to γ-rays. The main difference is their origin; γ-rays originate from the nuclei of decaying radio-isotopes, X-rays from electrons in X-ray tubes.
X-ray tubes are vacuum containers with a heated cathode and a metal anode. The cathode emits electrons by thermionic emission, where heat allows electrons to escape their circuit into the vacuum. They accelerate towards the positively charged anode, gaining kinetic energy. Collision with the anode causes rapid deceleration, releasing kinetic energy as Bremsstrahlung (German for ‘braking energy’), which are X-rays. The anode can be tungsten, molybdenum or copper, all of which have high melting points. 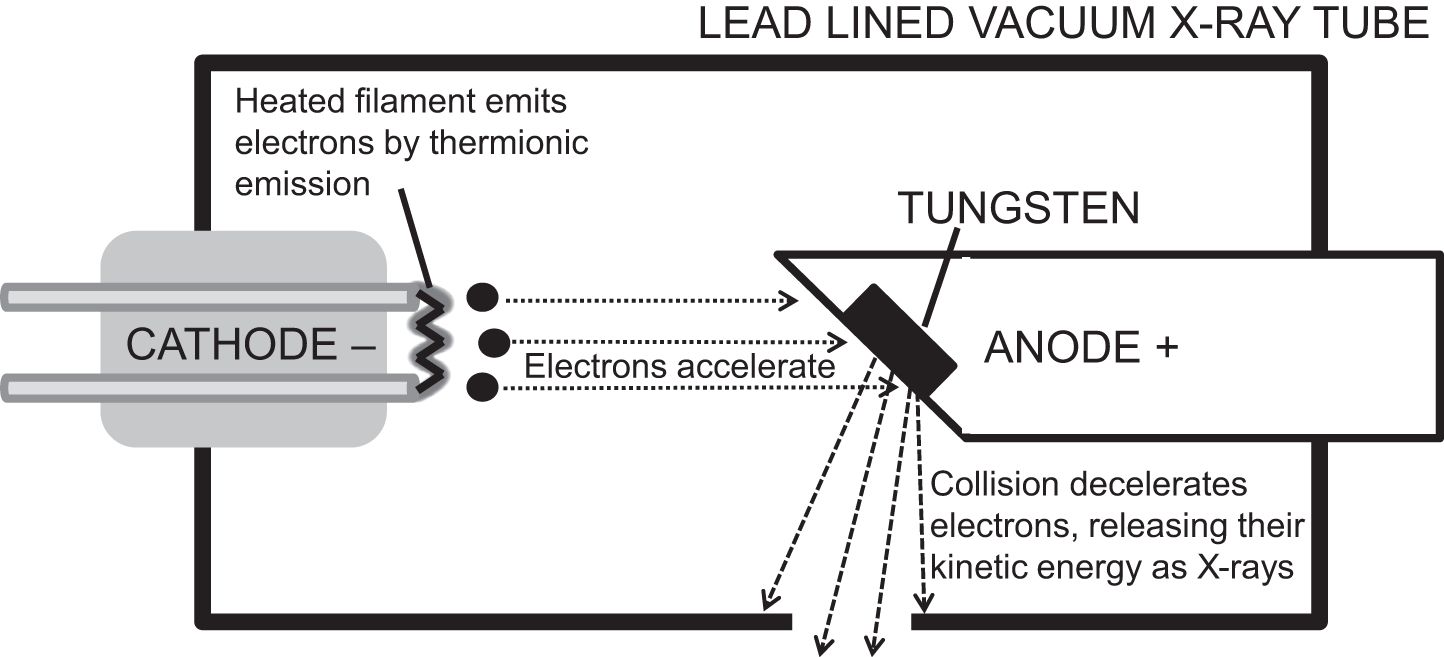
X-ray machines are rated according to voltage and current. Voltage is usually 30–150 kV, and determines electron energy. This influences wavelength and amplitude (thus penetration) of the X-rays produced. Current is usually 1 mA–1 A, and determines frequency of electron emission from the cathode. This affects the number of X-rays produced per second, which influences image resolution and total dose.
Geiger Muller tubes or scintillation counters can detect radiation. Geiger Muller tubes comprise an inert gas-filled chamber containing helium, neon or argon, with a high voltage applied across it. Radiation ionises the gas, allowing current to flow in proportion to the amount of radiation present. Scintillation counters use sodium or caesium iodide. Radiation striking these substances causes emission of electromagnetic waves of light (scintillation), which is detected by a photomultiplier tube and converted into a reading corresponding to the amount of radiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





