ION CHANNELS AND PAIN
A wide variety of voltage- and ligand-gated ion channels are needed for the generation and propagation of neuronal action potentials. Many of these channels are generically expressed by a wide variety of neurons. Certain ion channels, however, appear to be localized to neurons conveying nociceptive information. Such channels are potential therapeutic targets; selective blockade could produce analgesia. An understanding of the ion channels expressed on first order Aδ– and C-fibers, as well as second and third order neurons in the dorsal horn and thalamus is obviously of considerable interest to pain research. This chapter is limited to a discussion of voltage-dependent calcium and sodium channels, as well as certain members of the families of potassium and acid-sensing channels, the TRP family, and purinoceptors implicated in pain transmission. Throughout, an emphasis will be placed on studies examining intracranial pain.
VOLTAGE-DEPENDENT CALCIUM CHANNELS
Voltage-dependent calcium channels (VDCC) are composed of
α1,
α2δ, β, and
γ subunits; the
α1 subunit contains the selective calcium ion pore, a voltage sensor, and the binding site for selective high-threshold VDCC blockers. Ten isoforms of the
α1 subunit have been identified and this forms the basis for the most recent classification system (
52). The 10 isoforms are grouped into three families according to their sequence homology (
Table 20-1). VDCCs have also been grouped into six classes: L, N, P, Q, R, and T according to their electrophysiologic properties and pharmacologic susceptibility to specific blocking agents. At the most basic level, voltage-gated calcium channels may be divided electrophysiologically into two groups depending on their threshold of activation. Low-threshold VDCCs (T-type VDCCs) are activated at more hyperpolarized membrane voltages relative to the high-threshold (L-, N-, P-, and Q-type) channels (see references in Catterall ([
22]).
VDCC are differentially distributed at the neuronal level. P/Q-type VDCCs are located predominantly at presynaptic sites (
174,
175), N-type are found both pre- and post-synaptically (
173), and T- and L-type channels are mainly located on the proximal dendrites and soma of neurons (
33,
69,
172). Synaptic release of neurotransmitters depends on the influx of calcium ions through voltage-gated calcium channels. P/Q-type VDCCs appear to be the most prevalent exocytotic calcium channel within the central nervous system (CNS) (
49) controlling the release of excitatory amino acids, monoamines and peptide neurotransmitters (
95,
127,
159,
163). Exocytotic release of a variety of neurotransmitters is also inhibited by the N-type blocker
ω-conotoxin GVIa (
45,
95,
147,
159,
163). Excitatory glutaminergic neurotransmission is not completely prevented by blockade of N-type channels, indicating that several VDCCs are involved in neurotransmitter release (
159,
163). Antagonism of one type of calcium channel may therefore not significantly affect sensory signaling, especially if the presynaptic neuron is strongly depolarized. L-type channels do not appear to have a very significant role in neurotransmitter release in the CNS (although the nature of the stimulus used to evoke neurotransmitter release may be crucially important; nifedipine may inhibit release of substance P from dorsal root ganglion cells when they are depolarized with KCl but not electrical stimulation [
73]). L- and N-type channels may have a more important role regulating neuronal membrane properties and synaptic integration. In the dorsal horn of the rat, just as in other neuronal systems such as cortical and hippocampal pyramidal cells, high-threshold calcium currents can generate both regenerative and plateau potentials (
77,
142,
166). Plateau potentials sustained by calcium currents may result in a shift of the resting membrane potential toward threshold levels and contribute to the non-linear response properties observed in dorsal horn neurons (
109). The response of a dorsal horn neuron to nociceptive stimulation may therefore be dramatically enhanced
following activation of calcium (possibly L-type VDCCs) mediated plateau potentials (
108). Calcium-channel conductance is also subject to the modulatory effects of various neurotransmitters and peptides, which can further affect a neuron’s response characteristics at any given voltage (
48,
84,
112).
It is unsurprising that VDCCs may be targets for compounds with potential analgesic properties. Gabapentin, a drug commonly used for the treatment of neuropathic pain, is also used as a prophylactic treatment for migraine. This calcium-channel modulator inhibits high-threshold VDCCs in dorsal root ganglion neurons (
157) and may influence excitatory and inhibitory neurotransmitter release in the spinal dorsal horn (
8). The N-type channel blocker ziconotide has also shown promise in clinical trials as a treatment for postoperative and cancer pain (
155). Difficulties arise, however, when trying to determine pharmacologically the role of VDCCs in pain pathways. As we shall see, the effects of blocking individual channels may differ depending on the type of drug used in each study, as well as the dosage and their route of administration. The nature of the nociceptive stimulus may also crucially influence the outcome (for comprehensive reviews see Vanegas and Schaible [
164] and Prado [
129]). It is not clear, for example, whether results from models of chronic inflammatory and neuropathic pain are equally applicable to intracranial nociceptive neurotransmission, although it is reasonable to assume a degree of commonality.
L-Type VDCCs
Although blockers of L-type VDCCs have demonstrated analgesic properties against nociceptive stimuli (
39,
70,
97,
110,
116,
162), negative results have been reported as well (
97,
151,
152). Behavioral studies on mice in which the gene encoding the Ca
V1.3 (
α1D) subunit has been ablated do not appear to support a role for L-type channels in acute thermal nociceptive signaling (
28). Visceral pain transmission may rely in part on L-type channels; several studies show an analgesic effect for selective antagonists (such as verapamil and diltiazem) (
39,
70,
105); however, this has not been universally demonstrated (
74). L-type channels have also being implicated in inflammatory pain signaling, at least in models that use chemical irritation of peripheral nerves and joints as a nociceptive stimulus. In these models, two behavioral phases are noted, an early and a late phase, the later of which correlates with the onset of central sensitization (
44). Although L-type VDCCs have a doubtful role in the early phase, they do have a modest effect reducing behavioral responses associated with the late inflammatory phase (
29,
97,
116).
N-Type VDCCs
Blockers of N-type channels have demonstrated analgesic properties against acute mechanical and thermal nociceptive stimulation in several animal studies (
97,
115,
116),
although again these results have not been replicated in all experiments (
151). Preliminary clinical data further suggest that N-type channels may be important in human pain pathways. The selective blocking agent ziconotide has analgesic properties when administered intrathecally (
5,
155); however, side effects in the initial titration period remain a serious problem. N-type channels also have an important function in chronic inflammatory and neuropathic pain. Upregulation of the Ca
v2.2 subunit is correlated with pain behavior following neural or chemical injury (
25,
180). The delayed response to inflammatory agents is attenuated by treatment with N-type blockers (
41,
103,
115,
116,
151,
152) and they are still effective at reducing nociceptive behaviour, even if central sensitization has become established. The advent of genetically modified mice lacking functional N-type channels confirms their importance in the development of chronic pain states (
144). Studies on the behavioral responses elicited by acute noxious thermal and mechanical stimulation, however, have yielded conflicting results (
71,
86). In general these mice do not manifest significantly altered behavioral responses, which questions their role in the transmission of acute pain (
111,
145).
P/Q-Type VDCCs
Because P/Q-type channels are involved in both excitatory and inhibitory synaptic neurotransmission (
49,
95,
159,
163), it is not surprising that P/Q-type channel blockers are reported to have inhibitory, facilitatory, or even no effects on the responses of spinal neurons to nociceptive stimulation (
110,
114,
151,
152). It is probably simplistic to think that P/Q-type channels are only involved in excitatory mechanisms of pain transmission. Behavioral studies using natural mutant
“leaner” mice confirm that mutations of P/Q-type channels may modulate noxious sensory information in complex ways. Although analgesic behavior is demonstrated following mechanical testing, hyperalgesic responses are observed after noxious thermal stimulation (
121). It is also becoming apparent that P/Q-type channels have important actions in GABAergic inhibitory circuits. When mutant Familial Hemiplegic Migraine type 1 (FHM1) P/Q-type channels are expressed in inhibitory interneurons, it has been reported that they are less able to sustain GABAergic synaptic currents (
17). Application of
ω-agatoxin GIVa to the brainstem leads to an increase in spontaneous firing of medullary dorsal horn neurons (while also inhibiting the responses to noxious stimulation of the dura mater), possibly because of an action on GABAergic interneurons (
50). A similar disinhibitory action may also be observed following micro-injection of P/Q-type blockers into the periaqueductal grey (PAG) (
88). P/Q-type channels may therefore have a role in both inhibitory as well as excitatory neurotransmission, influencing the gating of sensory information at multiple levels in the nervous system. P/Q-type channels also have a role in the perception of inflammatory pain. Primary and secondary hyperalgesia resulting from chronic inflammation is prevented by pretreatment with P/Q- blockers (
41,
97,
114,
151), which suggests that P/Q-type channels may have an important role in the development of central sensitisation.
R-Type VDCCs
Until recently, studying the role of R-type VDCCs was limited by a lack of specific blocking agents. Attempts have been made to circumvent this limitation by generating Ca
V2.3 (
α1E) knockout mice. Whereas both homozygous Ca
V2.3-
null and heterozygous mice exhibit normal responses to acute noxious thermal, mechanical, and chemical stimuli, homozygous mutant mice demonstrate reduced behavioral responses to somatic inflammatory pain. Heterozygotes but not Ca
V2.3-null mice also appear to have impaired responses following nociceptive stimulation of the viscera (
143,
145). Recently it has been suggested that the peptide SNX-482 may act as a relatively selective R-type blocker (
14). Intrathecal administration of SNX-482 appears to have complex actions on nociceptive behavior. In the formalin test the late-phase response is attenuated in a dose-dependent manner but the early phase is either unaffected or even potentiated (
110). At present it appears that R-type channels are involved in the development of somatic inflammatory pain and possibly also visceral pain, but it is not clear what—if any—role they play in the transmission of acute nociceptive information.
T-Type VDCCs
Blockade of T-type channels with ethosuximide reduced spinal dorsal horn neuronal firing in response to electrical, mechanical, and thermal stimulation in a dose-dependent manner in a model of neuropathic pain (
102). Such hyperalgesic behavior may be mediated in part by a synergistic interaction between T-type VDCCs and neurokinin 1 receptor activation in lamina I neurons (
80). In addition to mechanically induced neuropathic pain (
47), T-type blockers also appear to be effective in combating nociceptive behavior resulting from chemotherapy-induced neuropathy (
55). Further evidence supporting a role for these channels in nociceptive transmission is provided by the antisense targeting of Ca
V3.2 mRNAs. This results in a significant reduction of T-type channel currents with a concomitant antinociceptive effect in models of both acute and chronic somatic pain (
15). T-type currents also have a central function modulating thalamic neuronal firing. The transition from tonic to burst mode may have important sensory gating properties, regulating the flow of visceral nociceptive information (
87).
VOLTAGE-GATED CALCIUM CHANNELS AND INTRACRANIAL NOCICEPTION
Although it cannot be assumed that the same array of high-threshold VDCCs are involved in spinal and trigeminovascular nociceptive signaling, initial studies do indicate that N- and P/Q-type channels have a role in transmitting sensory information from the dura (
50). Blockade of N-type channels effectively inhibits the responses of neurons in the spinal trigeminal nucleus to cold and inflammatory stimulation of the dura mater. Blockade of P/Q-type channels have a less profound effect, whereas L-type block does not have a significant action. Interestingly in both cases application of L- and P/Q-type VDCC blockers produce an increase in spontaneous firing rates, suggesting a disinhibitory action on dorsal horn neurons. Because VDCC blockers were applied to the exposed brainstem and upper cervical cord, the possibility that these effects were the result of actions at other sites, such as in the PAG, cannot be excluded (
88). This problem of anatomic localization has been overcome by microiontophoretic ejection of VDCC blockers directly onto trigeminal neurons (
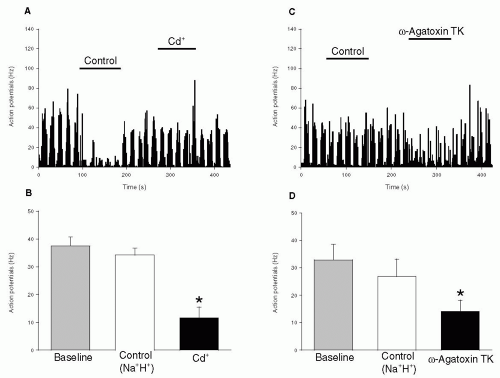
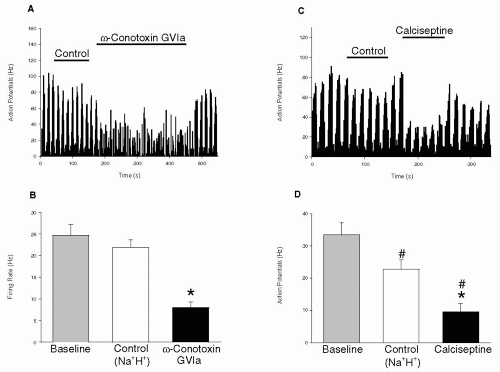
149). In this study L-, N- and P/Q-type channels were all shown to contribute to action potential generation by second order neurons in the trigeminocervical complex (
Figs. 20-1 and
20-2). Furthermore, neuronal firing triggered by electrical stimulation of the superior sagittal sinus (which is known to cause pain in humans) could also be inhibited by L- and N-type blockers (
Fig. 20-3). Although evidence for the effects of L-type channel blockers in the trigeminal nucleus is conflicting, their action on neurogenically induced dural vasodilation suggests that these channels may be involved in trigeminovascular nociceptive pathways in the periphery (
1). The potent L-type channel blocker calciseptine can inhibit the presynaptic release of CGRP from trigeminovascular neurons, as did P/Q- and N-type VDCC blockers.
Given the central contribution that VDCCc make toward action potential generation and synaptic neurotransmission, it is hardly surprising that VDCCs have an
important role in pain signaling. Blockade of individual channels may not necessarily prevent transmission of nociceptive information, but certain channels appear to serve a more integral part in pain transmission than others. All VDCCs appear to have complex actions, and in some cases this may involve modulating activity in GABAergic interneurons as well as descending inhibitory circuits. Whether this can be translated into further successful therapeutic interventions remains to be seen.