Invasive Fungal Infection
Toshibumi Taniguchi
Keith F. Woeltje
Invasive fungal infections are a significant cause of morbidity and mortality worldwide. The incidence of these infections is steadily increasing. In addition, strains resistant to many commonly used antifungal agents are becoming more prevalent.
Candida species are by far the most common fungal pathogens encountered in the intensive care unit. Candida is now the fourth most common cause of nosocomial bloodstream infection. The term invasive candidiasis comprises several conditions including candidemia, endocarditis, meningitis, and other forms of deep organ involvement (e.g., endophthalmitis and hepatosplenic candidiasis). The attributable mortality for an episode of invasive candidiasis has been reported to be as high as 40% to 50%.
Perhaps the strongest risk factor for invasive candidiasis is length of intensive care unit stay, with most studies revealing peak incidence at approximately day 10. Colonization with Candida (e.g., rectal, sputum, urine, or superficial wound colonization) is also considered a risk factor for the development of invasive disease. The importance of multifocal colonization remains an area of debate. Multiple single-center studies have suggested that multifocal colonization carries higher predictive value for development of invasive disease; however, one of the largest multicenter prospective trials done to date did not show a significant relationship between the number of colonized sites and the development of invasive disease. Other risk factors are listed in Table 39.1.
Candida albicans remains the most common species isolated from patients, accounting for 44% to 71% of disease. However, an epidemiologic shift toward non-albicans species is occurring, with the most common non-albicans isolates being Candida glabrata, Candida parapsilosis, Candida tropicalis, and Candida krusei. Recent data from the Prospective Antifungal Therapy Alliance (PATH) database from 2004 to 2008 showed the incidence of candidemia caused by the non-albicans Candida species was higher (54.4%) than the C. albicans (45.6%). This shift has particularly important implications for therapy because of intrinsic fluconazole resistance carried by C. glabrata and C. krusei.
The presence of Candida in a blood culture should never be perceived simply as a contaminant and should always prompt further investigation for possible sources. Although Candida grows readily in current blood culture bottles, blood cultures are positive in only 50% to 70% of patients with invasive candidiasis. However, a positive culture from a nonsterile site often provides little evidence to distinguish between infection and colonization. Biopsy of a specific lesion (from a normally sterile site) demonstrating characteristic histopathology can be considered definitive, but this is
often not feasible in critically ill patients. Because of these limitations, a reliable, nonculture-based method has been vigorously sought. 1,3-β-D-glucan, which is a major component of fungal cell wall, can be detected and its assay is recently approved by the Food and Drug Administration (FDA). This test has sensitivity of 75% to 100% and a specificity of 88% to 100%. However, it is a broad-spectrum assay that detects Aspergillus, Candida, Fusarium, Acremonium, and Saccharomyces species, thus careful interpretation is needed. None of the currently available tests have adequate sensitivity and specificity for reliable diagnosis. For this reason, empiric therapy for invasive candidiasis is often appropriate for the critically ill patient with risk factors who is not improving with appropriate antibacterial agents. Furthermore, recent evidence suggests a decrease in mortality when antifungal therapy is started early in high-risk hosts showing clinical signs of disease rather than holding therapy until definitive diagnosis (i.e., Candida growth in blood culture). Although no major conclusions can be drawn from this limited analysis, we believe that early empiric therapy in high-risk patients pending culture results is justifiable.
often not feasible in critically ill patients. Because of these limitations, a reliable, nonculture-based method has been vigorously sought. 1,3-β-D-glucan, which is a major component of fungal cell wall, can be detected and its assay is recently approved by the Food and Drug Administration (FDA). This test has sensitivity of 75% to 100% and a specificity of 88% to 100%. However, it is a broad-spectrum assay that detects Aspergillus, Candida, Fusarium, Acremonium, and Saccharomyces species, thus careful interpretation is needed. None of the currently available tests have adequate sensitivity and specificity for reliable diagnosis. For this reason, empiric therapy for invasive candidiasis is often appropriate for the critically ill patient with risk factors who is not improving with appropriate antibacterial agents. Furthermore, recent evidence suggests a decrease in mortality when antifungal therapy is started early in high-risk hosts showing clinical signs of disease rather than holding therapy until definitive diagnosis (i.e., Candida growth in blood culture). Although no major conclusions can be drawn from this limited analysis, we believe that early empiric therapy in high-risk patients pending culture results is justifiable.
TABLE 39.1 Risk Factors for Invasive Candidiasis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
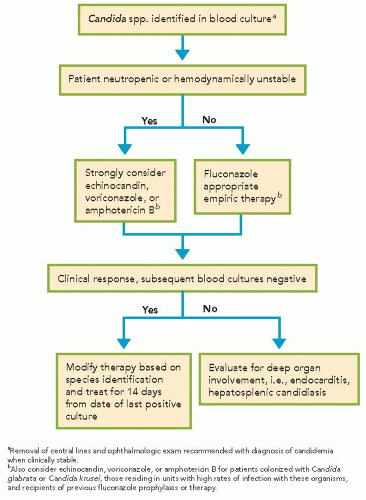
Evidence-based guidelines regarding general management and treatment of candidiasis were published by the Infectious Diseases Society of America in 2009. Fluconazole is an appropriate choice for nonneutropenic, hemodynamically stable patients unless there is high suspicion for a fluconazole-resistant species (e.g., previous colonization with C. glabrata, C. krusei, or history of recent azole exposure). If fluconazole is used for empiric therapy, a relatively high dose (e.g., 800 mg intravenously daily and adjusted for renal function) should be used until species identification is made. In patients who are neutropenic, hemodynamically unstable, or who are being treated in units with high rates of infection with fluconazole-resistant species (regardless of immune function or hemodynamic status), treatment with an echinocandin is preferred until species identification of the Candida isolate is made (Algorithm 39.1); amphotericin B formulation may be used as an alternative if there is intolerance to or limited availability of other antifungals. Voriconazole is another alternative, however, if offers little advantage over fluconazole and is recommended as step-down oral therapy for selected cases of candidiasis due to C. krusei or voriconazole-susceptible C. glabrata.
For infection due to C. parapsilosis, treatment with fluconazole is recommended since they are less susceptible to echinocandins. In the case of candidemia, the standard duration of therapy is 14 days fromthe last positive blood culture.Recommended treatment may be considerably longer, depending on the site of involvement. Early removal of the central venous catheter is recommended, but not all studies have shown that early removal is associated with clinical benefit; thus, timing of removal of central venous
catheter should be carefully individualized for each patient. Additional components of management include ophthalmologic examination to exclude endophthalmitis.
For infection due to C. parapsilosis, treatment with fluconazole is recommended since they are less susceptible to echinocandins. In the case of candidemia, the standard duration of therapy is 14 days fromthe last positive blood culture.Recommended treatment may be considerably longer, depending on the site of involvement. Early removal of the central venous catheter is recommended, but not all studies have shown that early removal is associated with clinical benefit; thus, timing of removal of central venous
catheter should be carefully individualized for each patient. Additional components of management include ophthalmologic examination to exclude endophthalmitis.
TABLE 39.2 Risk Factors for Invasive Aspergillosis | |||||||
|---|---|---|---|---|---|---|---|
|
Because fungal pathogens represent a growing proportion of nosocomial infections, prophylaxis with antifungal agents in high-risk patients has been implemented at several centers. The fewtrials performed to date have shown a nonsignificant risk reduction with the use of either fluconazole or ketoconazole as prophylaxis; however, these studies were limited by relatively small numbers. Although these results are encouraging, they have not led to definitive recommendations for antifungal prophylaxis. Antifungal prophylaxis may be a reasonable approach in selected high-risk patients such as transplant recipients or patients with chemotherapy-induced neutropenia.
Although a much less common cause of invasive disease, Aspergillus species remain an important consideration in a certain subset of patients, particularly those who are immunosuppressed (Table 39.2). Sinopulmonary involvement is the most common manifestation of invasive disease; however, dissemination can occur virtually anywhere, including the skin, central nervous system, eyes, and abdominal viscera. Computed tomography may suggest the diagnosis with findings such as the “halo sign,” a haziness surrounding a nodular pulmonary infiltrate. However, this feature can be seen with other angioinvasive infections, so it is far from diagnostic. The organism will grow on culture; however, its presence in samples fromnonsterile sites may indicate colonization rather than true infection. The sensitivity of the serum galactomannan assay varies widely in the literature between 29% and 100% and the specificity is typically greater than 85%. False-positive results may also occur in patients receiving piperacillin-tazobactam. β-D-glucan may be also useful to support the diagnosis of Aspergillus infection. These assays potentially can be used as a diagnostic adjunct but should not be used as sole criterion for diagnosis. Demonstration of the organism on biopsy is considered the gold standard for diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree