Hopefully by this point, you are starting to develop a much better sense of the principles at play when someone is on extracorporeal membrane oxygenation (ECMO). We started with the fundamentals, reviewing how oxygen is delivered throughout the body, how that system can break down, and the interventions to improve this delivery of oxygen, understanding that they can have dose-related toxicity that can compound with worsening respiratory/cardiac failure. We then introduced ECMO as a strategy that can mitigate that toxicity and were introduced to the components and configurations that we might come across. Finally, we explored the physiology of the various types of support, understanding the dynamics of flow, the capabilities of the membrane, and the rationale/limits of the various types of support.
This final section will now put attempt to put it all together, exploring the subtleties related to managing a patient on ECMO. The principles developed over the previous chapters will be fundamental, as they will form the foundation on which these management concepts are built.
The cornerstone of ECMO management: Lessen toxicity
Let’s return to our proposition that we are considering when it comes to the overall rationale of ECMO – namely that there are risks associated with ECMO; however, these risks can pay off in the long run if they mitigate the toxicity associated with conventional care ( Fig. 16.1 ).
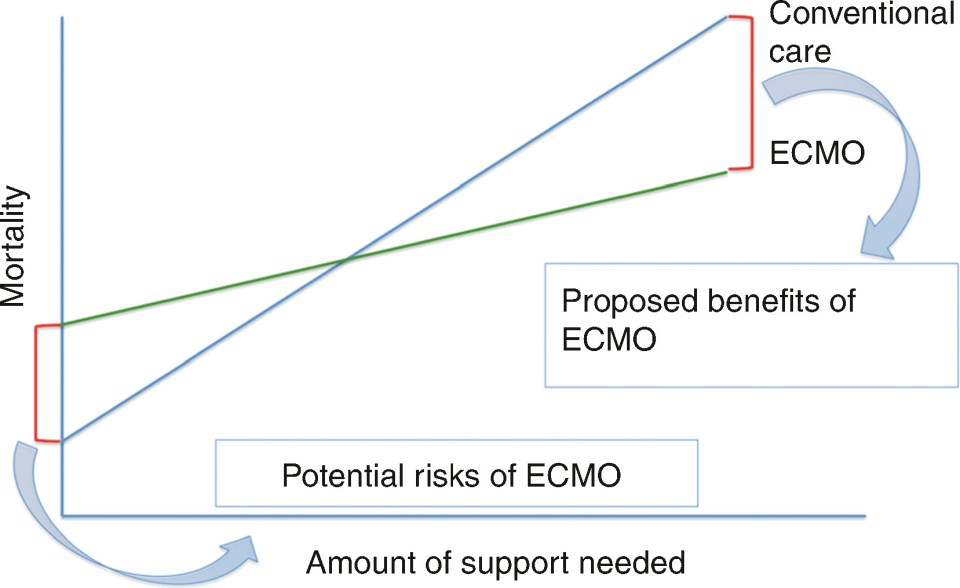
This proposition becomes the backbone of management when it comes to ECMO. Namely, ECMO only becomes advantageous to the degree that it mitigates the toxicity of support from conventional care.
What Is Meant by Toxicity of Conventional Care?
Let’s imagine a patient with no medical issues in the ICU with viral pneumonia and a superimposed bacterial pneumonia. He is intubated due to refractory hypoxia and is now on day 7 of his ICU course. The ventilator is clearly helping normalize his oxygen/CO 2 levels.
We often formulate our plan for how he will be managed by systems, from head to toe. Let’s now go head to toe and speculate on the potential damaging effects related to ICU support that he is at risk for that has nothing to do with his inciting injury.
Neurologic: He is likely on sedation to facilitate synchrony with the ventilator, which is making him more delirious. He may be developing post traumatic stress disorder that can be related to oversedation or undersedation. He is likely having sleep-wake disturbances. He may be on paralytics due to hypoxia, which is increasing his risk of weakness.
Pulmonary: The ventilator may be damaging his alveoli causing excessive stretch and barotrauma/volutrauma. Even at low, “lung-protective” ventilator settings, there may be some alveoli that are overfilled (leading to barotrauma/volutrauma), while some are underfilled (leading to atelectotrauma).
Cardiac: Positive end-expiratory pressure (PEEP) from the ventilator may impair his ventricular filling. Pressors may be worsening his ability to adequately deliver oxygen and worsen ischemia to end organs. The ventilator may be worsening his right ventricle.
GI: Medications and immobility may decrease his gastric motility. Tube feeds may be inadequate to meet his caloric demands. The ventilator and steroids administered may lead to stress ulcers.
Renal: Pressors and excessive volume administration may lead to volume overload. A urinary catheter to record urine output may be at risk of infection.
Endocrine: Medications and stress related to critical illness may worsen his glucose control.
Hematologic: Laboratory draws and volume administration may lead to anemia and potential need for transfusion.
Infectious disease (ID): Antibiotic administration may increase risk for antibiotic resistance and secondary infections after alteration of gut microbiome. He may be at risk of developing additional infections related to his central line and to the ventilator.
Musculoskeletal: He is at increased risk of progressive immobility and weakness. He may experience a loss in muscle mass and have progressive difficulty with recovery. This can be worsened in the setting of medications that can worsen myopathy (paralytics, steroids). Immobility may increase the risk of pressure ulcers and skin breakdown especially in the setting of suboptimal nutrition.
Social: Lack of access to familiar routines and loved ones may lead to depression. This may increase the risk of withdrawal and decreased desire to participate in care.
Will our patient develop all of these complications? Maybe not. In fact, many can be mitigated through attention to good, evidence-based critical care practices. Hopefully though, this list gives an appreciation of the risk that every patient experiences every day that they spend in our intensive care units. No wonder that we are so focused on getting people better and out of the ICU!
Does ECMO fix all these complications?
Certainly not. In fact, patients on ECMO may be at higher risk of some of these complications – infections and bleeding immediately come to mind. However, two points should be underscored by this example.
First, the degree of benefit of ECMO is directly related to what toxicity we can mitigate. This is the degree that the slope can be flattened of the green line representing care on ECMO in our graph. If placing someone on ECMO will allow for a significant reduction in ventilator settings, sedation, pressor requirement, and facilitate ambulation, the benefit of ECMO will be much higher than if a less profound impact can be anticipated.
Second, and just as important, the principle of reducing toxicity should inform the day-to-day management of patients on ECMO. Whenever possible, ECMO should be used to facilitate the reduction of toxicity of care, for example, preferentially optimizing the ECMO circuit to ensure the maintenance of lung-protective settings. The more this can be done, the greater the derived benefit of ECMO support.
Putting it together
At its best, good ECMO management translates into excellent critical care. Good critical care management is not only a prerequisite for caring for a patient on ECMO, but can also be facilitated while on ECMO in a way that cannot be facilitated in conventional care.
Whenever possible, use the advantages of being on ECMO to facilitate recovery and to implement best practice care for your patient. You can optimize blood flow to the circuit to maximize oxygen delivery, in order for excess sedation, analgesia, and paralytics to be removed. You can increase the amount of support to allow for maximal performance with physical therapy. You can adjust sweep gas flow to allow for lung-protective mechanical ventilation settings.
As such, we will not be spending a lot of time reviewing best practices in this section. To your best ability, treat infections, optimize nutrition, facilitate rehabilitation, mitigate delirium, and engage family/surrogates to best treat the patient. Whenever possible, ECMO should be used to further implement rather than to impede these goals.
Rather, the remainder of this section will focus on the management aspects unique to the care of patients on ECMO – flow/sweep titration, anticoagulation, mechanical ventilation techniques, and pharmacokinetics. Let’s now move ahead to further chapters to explore these unique attributes of management of patients on ECMO.
Suggested Reading
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








