Intravenous Regional Neural Blockade
Per H. Rosenberg
Intravenous (IV) regional neural blockade, or IV regional anesthesia (IVRA) is mainly used for short surgical procedures of the upper extremity. It can also be used for analgesia or anesthesia of the lower extremity, but certain toxicologic risks and methodologic circumstances make the use of IVRA of the lower extremity less attractive. In the last 10 years there has been very little change in this anesthetic technique itself (1,2,3); instead, in recent years, clinical research has focused on pharmacokinetics, toxicity, and benefits of additives in the local anesthetic solutions used.
History
Intravenous regional neural blockade was first described by the German surgeon August Bier in 1908 (4). The technique initially consisted of exsanguination of the arm by winding an Esmarch bandage from the fingers to above the elbow, application of two pneumatic tourniquets on the arm, one above the elbow and the other on the antebrachium, and then performing a surgical cutdown under local anesthesia in the cubital or antebrachial region to locate a superficial vein. A slit was made in the vein and a cannula was inserted in the peripheral direction and secured with a tie. The vein was ligated centrally to the slit.
After injection of procaine (0.25% or 0.5%), Bier noted a rapid initial analgesia in the region between the tourniquets, sufficient for starting the surgical procedure. A slower developing sensory block was noted in the region distal to the peripheral tourniquet.
Harvey Cushing introduced the pneumatic tourniquet, an adaptation of the original sphygmomanometer, in 1904. This device contained a hand pump, similar in design to that of bicycle pumps. Later, a manometer was added and the pump replaced with compressed pipeline air.
Mechanisms Of Action
The mechanisms of action in intravenous regional neural blockade are multiple and depend primarily on ischemia and on the transport of local anesthetic solution through a venous network into veins inside nerve trunks.
The nerve trunks of the extremities are composed of bundles covered with a connective tissue layer called epineurium, which also contain the blood vessels that supply the nerves. The endoneurium encloses the individual nerve fibers and contains capillary plexuses that extend interneurally as vasae nervorum.
Nerve fibers at the center of the fascicle are more distant from the lipoprotein-containing epineurium, and they are not protected by a strong diffusion barrier between the vessels and the nerve axons. Therefore, this is likely the reason for the commonly noted centripetal progression of the sensory block in IVRA.
Nerve endings in the skin are easily reached by the local anesthetic solution through valveless venules. The intercostobrachial nerves are not near a rich vessel network; that is, they cannot be rapidly reached by intravenously injected local anesthetic solution, therefore, their innervation region may sometimes be insufficiently blocked.
Surgical anesthesia in IVRA is produced by multiple and complementary mechanisms of action (Table 15-1). Initially, sensory block of the skin occurs along the veins (venules) filled with local anesthetic, a result of nerve conduction blockade of small nerves and nerve endings (peripheral mechanism of action). In the radiographic study by Fleming and co-workers (5), the development of skin analgesia followed the distribution of the injected mixture of local anesthetic and contrast medium. On the other hand, others have demonstrated an accumulation of injected contrast medium (6,7) or radioactive lidocaine (8) in the vicinity of the major nerves near the elbow in IVRA of the arm. When distal flow of a solution of local anesthetic and contrast medium was prevented by a tourniquet, anesthesia developed also in those parts peripheral to the distal tourniquet (7). This phenomenon was described by August Bier in 1908.
The uptake of lidocaine into the main nerves occurs rapidly (2–4 minutes), as shown by positron emission tomography, after the injection of [11C]lidocaine for IVRA (8). After tourniquet release, positron emission in the nerves decreased rapidly. More lipid-soluble and strongly protein-bound local anesthetics, such as bupivacaine and etidocaine, stay in the nerves longer, which in IVRA has resulted in prolonged analgesia after the deflation of the tourniquet cuff (9,10).
As long as the pressure in the tourniquet cuff stays low, nerve conduction block in IVRA through direct nerve damage probably plays a minor role (11). Ischemia, on the other hand, plays a major role, and total conduction block of the arm ensues after 15 to 45 minutes of ischemia (12,13,14). In conditions similar to those in a clinical IVRA of the arm, injection of saline instead of local anesthetic in volunteers resulted in complete sensory block of the skin with paralysis of the arm in 20 to 25 minutes (15).
Equipment
Equipment needed for IVRA are a pneumatic tourniquet, a pressure regulator with pressure gauges, an elastic bandage for exsanguination, an IV cannula in the extremity to be anesthetized, and syringes containing local anesthetic solution.
Table 15-1 Multiple mechanisms of action of intravenous regional anesthesia | |
|---|---|
|
Tourniquets
Soft padding on the extremity under the tourniquet is required. This is usually accomplished by applying either cast padding or tubular cotton stockinette, placing it as wrinkle-free as possible against the skin.
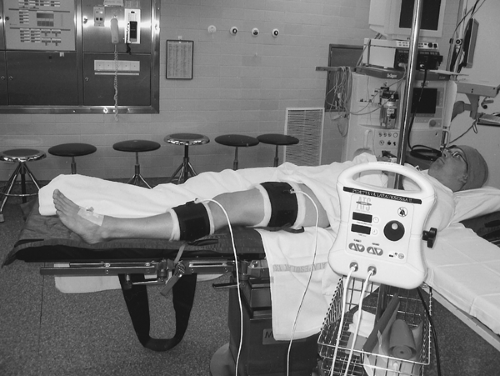
Tourniquets of different length and width are available for IVRA of the arm, with specially designed dual-bladder cylindrical or contour cuffs and with Velcro fasteners. Currently, the newest models are latex-free. The contour cuffs are particularly suitable in muscular or very obese adults. Two separate tourniquet cuffs may be used on the upper arms of very large patients, providing that enough room remains between the elbow and the axilla for two 7- or 9-cm wide cuffs. For IVRA of the whole lower extremity, two separate tourniquet cuffs fit well on the thigh. On the other hand, for IVRA of the leg below the knee, a single-cuff tourniquet is often applied on the calf, clearly below the head of the fibula (Fig. 15-1), with an unpressurized safety tourniquet in place on the thigh. In athletic patients, the calf tourniquet should be of the contour design and as wide as possible to diminish discomfort and tourniquet pain.
To prevent nerve injuries from the tourniquet, is important to minimize the destructive effect of the cuff pressure, especially the shear stress under the cuff edges. The pressure should be kept as low as possible; the wider the cuff, the lower the minimal limb blood flow occlusion pressure. For IVRA of the arm, with the tourniquet on the upper arm, a cuff pressure of 50 to 100 mm Hg above the systolic arterial blood pressure (i.e., 200–275 mm Hg in adults) is needed (16). For a thigh tourniquet cuff in adults, at least 100 mm Hg above the systolic arterial blood pressure (i.e., 250–300 mm Hg) is needed.
When the tourniquet cuff is inflated, several destructive mechanical and metabolic events are initiated (11). The tourniquet cuff itself, and in particular its edges, compresses the underlying nerves, disturbing both their structure and perfusion. Prolonged nerve conduction times at this region can be detected in 5 to 10 minutes (17,18). After about 30 minutes of compression at 300 mm Hg, a conduction block develops distal to the cuff. Tourniquet release resulted in recovery in about 5 minutes, but the conduction time at the level of the tourniquet, particularly across the proximal border region, was the last to recover (17). Interestingly, only 75% recovery of nerve conduction velocity was observed across the tourniquet zone 1 hour after the release of the cuff.
The metabolic changes include hypoxia, hypercapnia, metabolic acidosis, lacticemia, and loss of muscle cell membrane integrity. As a result, a local (isolated limb) and systemic inflammatory response (19) involving fibrinolysis (20) ensues.
When the tourniquet is inflated for a long time, a risk arises for the development of a reperfusion syndrome. In fact, the muscle tissue is quite vulnerable, with a critical tissue ischemic time of 4 hours (21). It may be of note that reversible histologic, ultrastructural, and neuromuscular deteriorating changes can
be observed after 1.5 to 2 hours of ischemia (22,23), and irreversible muscle cell damage starts after 3 hours of ischemia (24). Such studies have been guides to the determination of the safe duration of the application of an inflated tourniquet in clinical patients (i.e., 1.5–2 hours). In an awake patient (e.g., in IVRA), tourniquet pain usually limits the time to 1 hour.
be observed after 1.5 to 2 hours of ischemia (22,23), and irreversible muscle cell damage starts after 3 hours of ischemia (24). Such studies have been guides to the determination of the safe duration of the application of an inflated tourniquet in clinical patients (i.e., 1.5–2 hours). In an awake patient (e.g., in IVRA), tourniquet pain usually limits the time to 1 hour.
Exsanguination
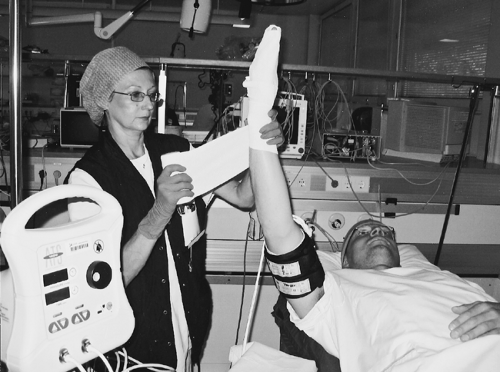
The extremity is best exsanguinated by winding an elastic rubber Esmarch, Martin, or other elastic bandage tightly from the periphery to the tourniquet (Fig. 15-2). August Bier was the first to apply this bandage in IVRA, but initially, both Johannes von Esmarch and Henry A. Martin used their elastic rubber bandages as a method of applying diffuse pressure on the entire lower extremity for the treatment of stasis ulcers and to prevent reoccurrence of effusion after aspiration of the knee joint.
In comparison of various techniques of exsanguination of the arm in volunteers, the Esmarch bandage reduces blood volume of the arm on average by 69% (25), whereas the reduction was only about 45% by elevation of the arm (Table 15-2). In patients with a traumatized arm, an elevation/arterial compression (brachial artery) exsanguination technique results in sufficient emptying of the veins for IVRA (26). The degree of emptiness of the veins is probably related to the evenness and efficacy of the distribution of the injected local anesthetic but this does not seem to affect the development and success of anesthesia of the arm (27). Overfilling and distention of the veins may result in swelling of the tissue structures and oozing of blood during surgery, which may be so disturbing that some hand surgeons do not allow anesthesiologists to use IVRA in their patients. This problem may be overcome by using exsanguination with an Esmarch bandage and injecting only moderate volumes of anesthetic solution (20–30 mL) for IVRA of the arm, or by using the so-called “second-wrap” technique (described later).
Today, other types of elastic bands (e.g., bands using elasthane or crepe), 6 to 12 cm wide, have replaced the classic rubber bandage. However, their efficacy in emptying the limb of blood without damaging the skin must still be confirmed.
Pressure Gauge
Tourniquet-induced muscle and nerve damage (tourniquet paralysis) was a great problem when the rubber bandage itself
was used as a tourniquet (11,28). Uncontrolled very high pressures were created under the bandage. Thus, the development of pneumatic tourniquets with pressure gauges connected were of great significance, perhaps more so for the safe conductance of extremity surgery.
was used as a tourniquet (11,28). Uncontrolled very high pressures were created under the bandage. Thus, the development of pneumatic tourniquets with pressure gauges connected were of great significance, perhaps more so for the safe conductance of extremity surgery.
Table 15-2 Efficacy of exsanguination of the upper extremity by various methods, as studied with a scintigraphic technique | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Modern pressure-control devices (tourniquet control units; Fig. 15-1) contain two different regulators and gauges, one for either of two tourniquet cuffs. In addition, these units contain alarms for time limits and self-regulation systems for maintaining either a set pressure or the limb occlusion pressure (LOP). In the IVRA technique, pressures slightly higher than LOP should be used, as recommended earlier, because of the risk of IV leakage of local anesthetic during and immediately after injection.
Drugs For Intravenous Regional Anesthesia
Lidocaine and Prilocaine
All available local anesthetics have been used for IVRA (Table 15-3). In principle, the least toxic local anesthetic should be chosen because the dose initially injected intravenously either in the upper or lower extremity is large enough to cause severe systemic intoxication if the dose mistakenly enters the circulation directly. Thus, the amide-linked local anesthetics lidocaine and prilocaine have been popular and safe drugs in IVRA. In countries in which prilocaine has not been available, lidocaine has been the first choice. Because of practical reasons determined by the use of a tourniquet, the onset of the IV neural blockade should be rapid; therefore, solutions of 5 mg/mL (0.5%) are commonly used. A few minutes may be won by using higher concentrations, but with the expense of causing toxic damage to the endothelium of the veins (39). In adults, the volume injected should, in principle, replace the volume removed by exsanguination; that is, 32 mL in women and 63 mL in men, on average (26). In an earlier study of adults, it was shown that 20 to 50 mL would suffice to fill the venous channels of an exsanguinated arm (40). The commonly used volumes for IVRA of the upper extremity in adults are 40 to 50 mL. The volume to fill the veins in an exsanguinated whole lower limb would probably be at least double these amounts, and in clinical practice, volumes of 100 to 120 mL have been applied (41). In the comparative study of the use of 6 mg/kg of either lidocaine or prilocaine, the plasma concentrations after cuff deflation (Fig. 15-3), as well as the incidence of central nervous system (CNS) toxicity symptoms were clearly lower when prilocaine was used, again emphasizing the advantage regarding the safety of prilocaine when large doses are applied. In a clinical situation, such a high dose of lidocaine is not recommended. As the concentration of the local anesthetic in the injected solution for IVRA of the whole lower extremity needs to be kept low, the onset of anesthesia is sometimes disturbingly long. The high pressure in the thigh tourniquet (often as high as 300 mm Hg) and the relatively slow onset of analgesia make the IVRA technique of the whole lower extremity less attractive in comparison with spinal anesthesia or peripheral nerve blocks. However, IVRA below the knee, or IVRA around the knee (discussed later) are interesting alternatives, lacking some of the problems linked to whole lower extremity IVRA.
Table 15-3 Local anesthetics used in intravenous regional anesthesia | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
With prilocaine 6 mg/kg, a moderate rise in methemoglobin (met-Hb) levels was noted after IVRA of the whole lower extremity (Fig. 15-4), albeit without clinical signs of cyanosis or hypoxemia (41). It has been shown that two metabolites of prilocaine, o-toluidine and nitrosotoluidine oxidize ferrous iron in the hemoglobin molecule to the ferric state (42). The interindividual variation in met-Hb concentrations after prilocaine anaesthesia are huge (41,42). The maximum dose recommendation of 600 mg prilocaine in adults for the avoidance of clinically serious methemoglobinemia is more circumstantial than evidence-based (43). The prediction of the occurrence of this side effect/complication is hampered also by the fact that exposure to several other drugs and chemicals (e.g., chloroquine, primaquine, glyceryl trinitrate, sulfonamides, phenacetin) may induce met-Hb of varying degree (44).
More concentrated prilocaine solutions (7.5–20 mg/mL) have been used for IVRA of both the upper and lower extremity (45,46). The onset of the neural blockade is shorter, but otherwise the blocking profile is very similar to that of IVRA with prilocaine 5 mg/mL. The occurrence of mild symptoms of CNS toxicity is more frequent with the more concentrated solutions if the dose is not reduced.
Mepivacaine
Bupivacaine and Analogues
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree