36 h) and VIII (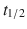 10–14 h) are labile and are the factors most affected during storage of plasma. Factors V and VIII also appear to be most commonly deficient in patients with trauma associated coagulopathy [15]. As a result of these findings and more recent studies demonstrating morbidity and mortality benefits, early treatment of severe trauma associated coagulopathy by resuscitation of blood products in a 1:1:1 ratio of PRBCs, FFP, and platelet units is recommended [16, 17].
10–14 h) are labile and are the factors most affected during storage of plasma. Factors V and VIII also appear to be most commonly deficient in patients with trauma associated coagulopathy [15]. As a result of these findings and more recent studies demonstrating morbidity and mortality benefits, early treatment of severe trauma associated coagulopathy by resuscitation of blood products in a 1:1:1 ratio of PRBCs, FFP, and platelet units is recommended [16, 17].
- 13.
What is activated Factor VII?
Recombinant activated human Factor VII (rFVIIa) can be administered in situations where Factor VII deficiency is suspected to contribute to coagulopathy. Activated Factor VII was initially developed and approved for use to treat hemophilia in patients who have developed inhibitors to Factors VIII or IX, acquired hemophilia, or individuals with isolated Factor VII deficiency. Since its introduction, however, several off label indications have emerged. In particular, given the short half-life of Factor VII and its synthesis by the liver, rFVIIa has been administered to correct coagulopathy in patients with liver dysfunction, especially those who may be unable to tolerate large volume loads from FFP transfusion. Additionally, rFVIIa has been used to reduce microvascular bleeding after trauma and surgery that is not responsive to standard blood product transfusion.
- 14.
What are fibrinogen degradation products and what do they affect?
When fibrin polymers are cleaved by plasmin, fibrin degradation products are released. Tissue plasminogen activator initially activates plasminogen to form plasmin. Plasmin is the primary enzyme responsible of fibrinolysis. In addition to fibrin, plasmin also degrades fibrinogen, and other coagulation factors. Its action on fibrin polymers liberates dimerized D-domains of fibrin into the circulation. The crosslinking of D-dimers is the result of Factor XIII activity during the process of fibrin clot formation. As a result, an increase in circulating D-dimers suggests fibrinolysis of an intravascular clot.
- 15.
What are the indications for transfusion of FFP?
According to the updated ASA Practice Guidelines on Perioperative Blood Management [18], plasma should be transfused in the following situations:
Excessive microvascular bleeding in the setting of an INR greater than 2.0 without the presence of heparin.
Excessive microvascular bleeding due to suspected clotting factor deficiency in patients transfused greater than 1 blood volume (when coagulation studies are not readily available).
Urgent reversal of warfarin when prothrombin complex concentrates are unavailable.
Correction of known clotting factor deficiencies when specific factor concentrates are unavailable.
Notably, plasma transfusion is not indicated when PT/INR and aPTT are normal, nor is it indicated to increase intravascular volume.
- 16.
How is cryoprecipitate made and what coagulation factors does it contain?
Cryopreciptate is generated by thawing fresh frozen plasma at 1–6 °C. Proteins insoluble at cold temperatures precipitate and can be collected via centrifugation. This precipitate is resuspended in a small amount of plasma (usually 10–15 mL). Each unit contains 200–250 mg fibrinogen, ≥80 IU FVIII, 80–120 IU vWF, 40–60 IU FXIII, and fibronectin. The usual adult dose is 10 units often administered as a 10-pack. Cryoprecipitate is a poor source of vitamin K-dependent factors (II, VII, IX, X). Thus, cryoprecipitate should not be used to reverse warfarin-induced anticoagulation; instead, FFP should be used. Similarly, cryoprecipitate should not be used to treat Hemophilia B (FIX deficiency); instead, recombinant human factor IX (rFIX) should be used.
- 17.
How can platelet activity be monitored?
Platelet activity is commonly monitored by aggregometry. This process involves incubation of a platelet suspension (platelet-rich plasma) with specific agonists such as collagen, ADP, epinephrine, or ristocetin. The time and degree of platelet aggregation is measured by monitoring light transmission through the suspension which increases as the platelets aggregate.
An alternative is the Platelet Function Analyzer, PFA-100. This test can be run on whole blood anticoagulated with citrate and stored at room temperature. Platelets within the blood are subjected to high flow rates within a capillary tube and are exposed to a collagen-coated membrane. An aggregation agonist (ADP or epinephrine) is added to provoke platelet aggregation. The resulting decrease in flow rate is monitored as a platelet plug is formed. This time is reported as the closure time (CT) and is prolonged in the setting of platelet dysfunction.
- 18.
What is the thromboelastograph?
The thromboelastograph (TEG) is an assay of the dynamics of clot formation from whole blood. The TEG device measures viscoelastic properties of the clot during formation and lysis which are then analyzed and plotted. Although TEG technology has been available for some decades, it has not yet established itself as a routine intraoperative analysis of coagulation. A recent Cochrane review found insufficient evidence to support use of TEG to guide transfusion practice in adult trauma patients; however, the authors note that this conclusion is likely due to a lack of consensus over how TEG measurements should influence patient management [19].
- 19.
What drugs could cause intraoperative coagulopathy?
The number of therapeutic drugs interfering with normal hemostasis is ever increasing.
Anticoagulants:
Vitamin K-antagonists—Warfarin reduces the production of essential factors such as II, VII, IX and X. Protein C and S concentrations, both anticoagulation factors, are also reduced.
Unfractionated heparin (UFH): UFH is a mixture of glycosaminoglycans of various lengths. UFH functions by complexing with antithrombin (AT, formerly known as antithrombin III) and accelerating its inactivation of thrombin and Factor Xa. UFH easily dissociates from AT upon protamine administration.
Low molecular weight heparin (LMWH): LMWH is a purified and smaller molecule than unfractionated heparin with greater anti-Xa activity and little inactivation of thrombin. LMWH has a longer half-life, which can increase with diminishing kidney function. Unlike UFH, LMWH cannot be completely reversed by protamine.
Fondapariunux and danaparoid are two LMWH-related compounds with higher specific anti-Xa activity and long half-lives.
Direct Xa inhibitors—Rivaroxaban (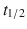 7–17 h), apixaban (
7–17 h), apixaban (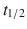 5–9 h), and edoxaban (
5–9 h), and edoxaban (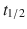 6–11 h) act to inactivate circulating and clot-bound FXa. They are administered orally and do not have a specific reversal agent.
6–11 h) act to inactivate circulating and clot-bound FXa. They are administered orally and do not have a specific reversal agent.
Thrombin inhibitors—There are many new formulations of compounds which inactivate circulating and clot-bound thrombin (factor IIa).
Parenteral—bivalirudin (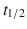 25 min), argatroban (
25 min), argatroban (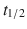 40–50 min), desirudin (
40–50 min), desirudin (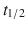 2 h)
2 h)
Oral—dabigatran (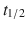 12–17 h); there is no reversal available for dabigatran.
12–17 h); there is no reversal available for dabigatran.
Antiplatelet Agents:
Aspirin—Aspirin irreversibly inhibits the platelet cyclooxygenase and thromboxane A2. Other non-steroidal anti-inflammatory drugs such as ibuprofen and celecoxib inhibit the cyclooxygenase in a mostly reversible fashion. The risk of significant intraoperative bleeding after aspirin or NSAIDs therapy is low.
ADP receptor blockers—These agents block the platelet P2Y12 receptor which is responsible for binding of adenosine diphosphate (ADP), thereby limiting platelet aggregation.
Clopidogrel, ticlopidine, prasugrel, ticagrelor, and cangrelor are examples of ADP receptor blockers.
GP IIb/IIIa Inhibitors—Agents in this class inhibit platelet aggregation by preventing crosslinking mediated by GP IIb/IIIa binding of fibrinogen.
Abciximab, tirofiban, or eptifibatide are currently used GP IIb/IIIa Inhibitors.
Medications with bleeding side effect:
Selective Serotonin Reuptake Inhibitors can reduce platelet activity by depletion of serotonin from platelet granules.
Valproic acid decreases levels of factors VII, VIII, XIII, platelets, vWF, fibrinogen, protein C, and antithrombin.
- 20.






