CHAPTER 27 Intradiscal Thermal Therapies
Description
Terminology and Subtypes
Intradiscal thermal therapies encompass a group of interventions that deliver heat energy to the intervertebral disc (IVD) with the goal of reducing discogenic pain by a variety of proposed mechanisms, including shrinking subannular disc protrusions, destroying nociceptors, sealing annular tears by collagen modification, and stimulating a healing response.1,2 The original intradiscal thermal therapies delivered heat to the nucleus using the same radiofrequency device used in lumbar medial branch neurotomy (LMBN), discussed in Chapter 26. Subsequently, the more widely used intradiscal electrothermal therapy (IDET) procedure used a catheter inserted into the nucleus and advanced circumferentially to the outer annulus. A later modification of the device used the same technique but a shorter active heating length using radiofrequency rather than electrothermal energy to heat the adjacent annulus. This procedure is also termed intradiscal electrothermal annuloplasty (IDEA) or intradiscal thermal annuloplasty (IDTA).
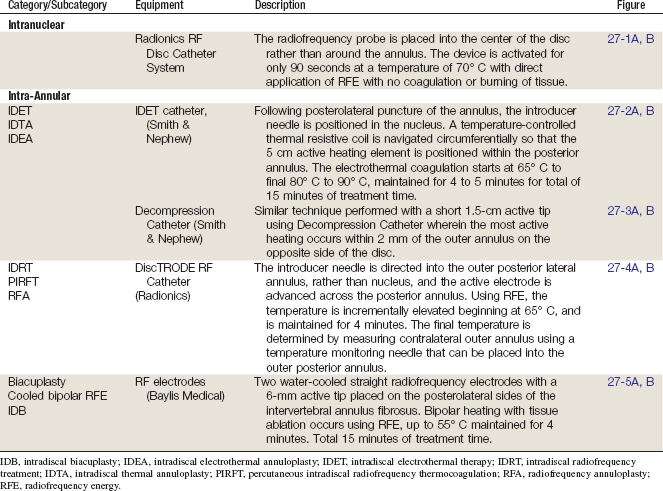
An overview of the various methods of intradiscal thermal therapies is presented in Table 27-1 and illustrated in Figures 27-1 through 27-5.
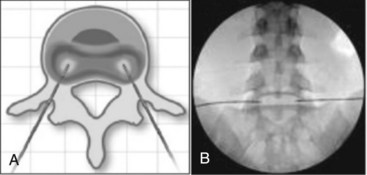
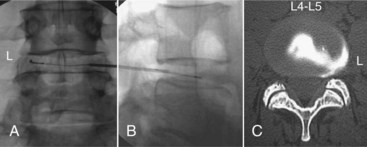
(A from: Finch PM, Price LM, Drummond PD. Radiofrequency heating of painful annular disruptions: one-year outcomes. J Spinal Disord Tech, Volume 18, Number 1, February 2005, pp 6-13. Courtesy Professor JA Taylor. B Courtesy of Baylis Medical Company, Inc. Images found at www.baylismedical.com/images/PDF%20pain/PM1007_TD.pdf.)
History and Frequency of Use
The rationale for heating IVDs was strongly influenced by preclinical and clinical studies investigating the application of heat to stabilize joints by modifying collagen. The intervention thermal capsulorrhaphy, which involves using laser or radiofrequency heating devices to denature the collagen of the shoulder capsule to cause shrinkage, has been used to treat shoulder instability since 1994.3 Type I collagen in the shoulder capsule has a triple-helical configuration that is responsible for the molecule’s ability to resist tensile forces, and is similar to the collagen found in the outer annulus of the IVD.
The biologic effects of laser thermal modification were investigated by Hayashi and colleagues, who used a YAG laser to deliver 250 J to the glenohumeral and patellofemoral joint capsules.4,5 Joint capsule thickness immediately increased but normalized after 60 days, while stiffness and strength initially decreased but normalized after 30 days. Histologically, collagen hyalinization and cell death was noted at the application site, but the surrounding tissues were normal. Healing was thought to be the result of an active reparative process initiated by acute inflammation resulting in fibroblast proliferation and migration, neovascularization, and eventual collagen repair. Biochemical analysis revealed that the laser treatment had denatured collagen by disrupting alpha bands, resulting in unwinding of the triple helix. Because the initial effects of laser energy were deleterious, recovery was dependent on a subsequent healing response. It should be noted that although cartilaginous tissue regained most of its preinjury strength, long-term weakness remained during creep strain.
The US Food and Drug Administration (FDA) gave clearance to the medical device used during IDET—the SpineCATH intradiscal catheter—in March 1998. It has been estimated that more than 40,000 procedures were performed in the first 5 years after IDET was approved for marketing.6 The manufacturer of the IDET device, Smith & Nephew (Memphis, Tennessee) has estimated that 60,000 procedures have been performed worldwide as of June 2005.6
Procedure
Catheter Placement
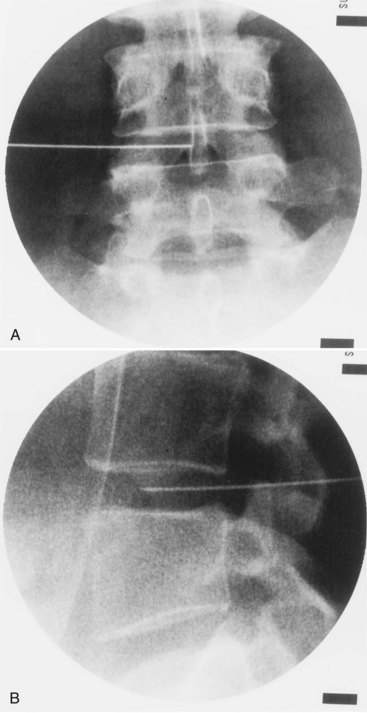
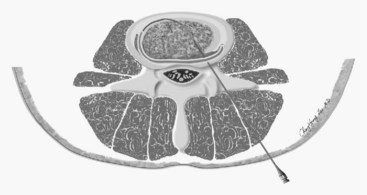
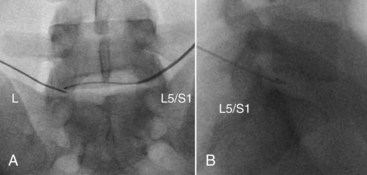
The IDET procedure uses a navigable intradiscal catheter with a thermal-resistive coil, the SpineCATH intradiscal catheter or decompression catheter. A 17-gauge introducer needle is first inserted through the skin, subcutaneous tissue, and muscle, and advanced circuitously to the posterior annulus; catheter position is critical to IDET.7,8 Using a standard posterolateral discogram technique, a 30-cm catheter with a 5- or 1.5-cm active electrothermal tip is then inserted through the introducer needle (Figure 27-6). One catheter is usually enough, but in some cases two catheters may be used in a bilateral deployment to treat the entire posterior annular wall.7 Ideally, the active length of the catheter should cross the “symptomatic” annular fissure and lie close enough (approximately 5 mm) to the outer annulus to allow sufficient heat to spread to both the outer and inner annulus (Figure 27-7).9
Heating Protocol
The original 5-cm active heating element catheter introduced by Oratec placed the temperature sensor electrode deep within the catheter and reported temperatures that were on average 13° C higher than those administered to tissue at the tip of the catheter. The empirically derived heating protocol recommended beginning at 65° C, with incremental increases of 1° C every 30 seconds to achieve a final temperature of 80° C to 90° C that should be maintained for 5 minutes; the total treatment time was approximately 15 minutes.10 Although some clinicians advocated using higher temperatures, there was no standard method for determining the actual final temperature delivered to targeted tissues.
Derby and colleagues were not able to demonstrate a correlation between higher final temperatures and improved outcomes with IDET after 8 or 16 months.11 In fact, when catheter positions were less than 5 mm from the outer annulus, higher temperatures were associated with longer postprocedure increased pain. Based on these results, it was proposed that temperatures should be incrementally increased to 80° C but that the final temperature and total duration of the intervention should be based on the patient’s pain response. In this scenario, incremental increases in temperature would be stopped when the patient reported pain rated higher than 6 to 8 out of 10. This temperature would then be maintained for up to 4 minutes as long as pain remained less than 8 out of 10.
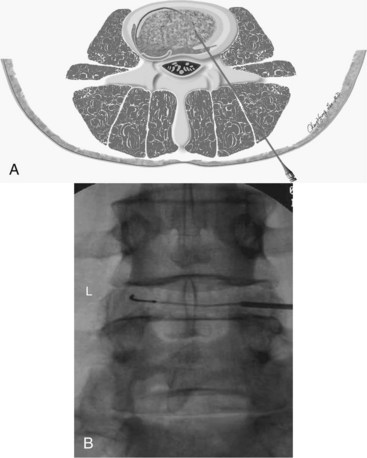
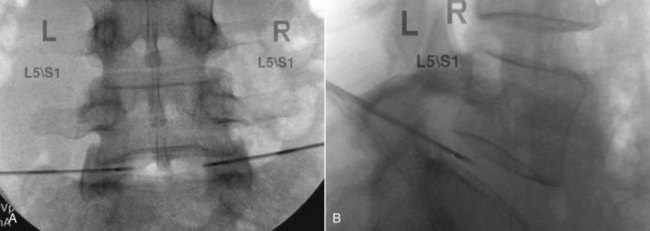
The newer IDET catheter (Decompression Catheter, Smith & Nephew) used a shorter 1.5-cm heating element, which was thought to help limit destruction of normal annulus tissue (Figures 27-8 and 27-9). Because the temperature sensor was placed on the outside of the catheter, measurements were more likely to reflect those of adjacent tissues. The heating protocol using the newer IDET catheter also called for beginning at 65° C, incrementally increasing temperatures 1° C every 30 seconds up to 90° C, which was to be maintained for 6 minutes. Because the newer catheter was shorter than the original, the total dose of heat delivered with this protocol was probably less. Anecdotally, some practitioners reported improved outcomes with shorter post-procedure pain and greater tolerance of increased temperature before patients complained of excessive pain when using the newer catheter.
Post-Procedure Care
Following the IDET procedure, the patient is typically monitored in a recovery room for 1 to 3 hours before discharge.8 Patients are warned to expect a significant increase in pain, and are told that this may last 2 to 7 days, although infrequently this may be permanent. Patients typically wear a lumbar brace for weeks to months after the procedure while healing of the outer annulus occurs to minimize the risk of reinjury from excessive disc loading during this period. Activities such as walking and pool exercises are encouraged, but aggressive physical therapy is usually not begun until 1 to 2 months after the procedure.12 Most patients have reached maximal improvement by 3 months but a minority may take up to 3 to 6 months.9,12–14
Intradiscal Radiofrequency Treatment
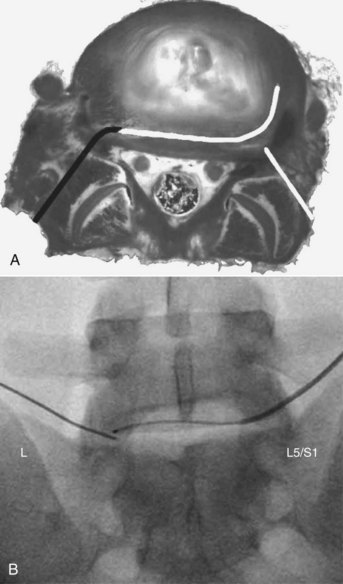
This approach is similar to IDET but rather than passing the introducer needle into the nucleus, the needle tip is placed directly into the outer posterior lateral annulus, and the active electrode is advanced across the posterior annulus (Figure 27-10). The device used for the procedure, the DiscTRODE radiofrequency catheter, is manufactured by Radionics (Burlington, Massachusetts) and includes a sensor that measures tissue resistance, detecting the higher resistance of the disc annulus with both sound and digital readings. By passing the needle tip slightly more posteriorly and medially in the annulus, an asymmetric opening in the needle tip helps direct the active electrode across the posterior disc annulus. The device also includes a temperature monitoring needle that can be placed into the outer posterior annulus to monitor the increase as the electrode is heated. A graduated temperature protocol beginning at 65° C is used, but the final temperature is determined by measuring that of the contralateral outer annulus. Although this is thought to represent a more accurate method of determining final temperature, the distance of the measuring electrode from the radiofrequency electrode varies. When using this device, the temperature is incrementally elevated to 65° C, which is maintained for 4 minutes. Similar post-procedure care is followed.
Intradiscal Biacuplasty
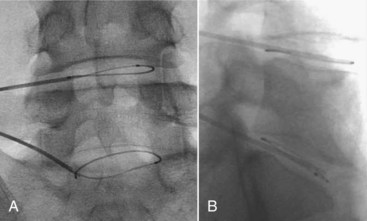
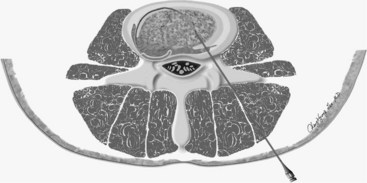
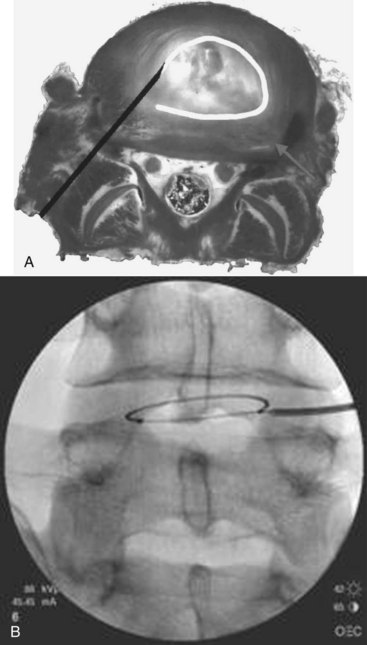
The IDB procedure is somewhat similar to the IDET procedure described above. However, the probes are heated to 45° C for 15 minutes (approximately 60° C to 65° C tissue temperature) while water is continuously circulated around the probes. The heating is typically less painful than heating using the IDET catheters. Disc IDB is a method of heating the outer annulus that uses a bipolar cooled radiofrequency system (TransDiscal System, Baylis Medical, Montreal, Quebec, Canada). The lesion is created between two water-cooled radiofrequency probes placed into the right and left outer disc annuli. Using a standard posterior lateral approach to the lumbar IVD, introducer needles are passed into the outer disc annulus from the right and left side. The stylettes are removed and radiofrequency probes are passed through the introducer needle to lie within the middle and inner annular fibers (Figure 27-11). By placing two electrodes in a disc in a bipolar arrangement, radiofrequency current is concentrated in the disc and creates a larger size lesion compared to standard radiofrequency electrodes.15 Water circulating in the electrodes cools the electrode surfaces, which allows for greater power delivery while at the same time not overheating tissue local to the electrodes. This allows a three-dimensional lesion to develop between the electrodes, which theoretically would be able to seal radial and circumferential fissures throughout the posterior annulus.
An animal study of the histologic effects and thermal distribution of disc IDB in an in vivo porcine model has shown that the IDB procedure achieves suitable temperatures for neural ablation in the disc (44° C to 55° C) while showing no evidence of damage to adjacent nerve roots.16 Pauza assessed temperature profiles created by IDB in human cadaver discs, showing safe temperatures below 45° C in the anterior disc and posterior longitudinal ligament, while posterior annulus fibrosus outer and inner two-thirds reached 54° C to 60° C, respectively.17 A cadaver study by Kapural and colleagues reported that measured temperatures across the posterior annulus using the IDB technique were sufficient to ablate nerves in the disc while maintaining safe temperatures in the epidural space and neural foramina.18
Regulatory Status
The FDA gave clearance to the medical device used during IDET, the SpineCATH intradiscal catheter, in March 1998, for the coagulation and decompression of contained herniated discs in patients with low back pain (LBP). Obtaining approval as a class II medical device mainly requires that the manufacturer submits evidence that the new device is substantially equivalent to a currently approved similar device. The IDB system was approved for use by the FDA in 2006, when initial spinal temperature measurements and histologic studies reported an appropriate safety profile.18
Theory
Mechanism of Action
Despite many in vivo and in vitro studies using human and animal models, the precise mechanism of action through which IDET can help patients with chronic low back pain (CLBP) is unclear. Various changes have been reported with electron microscopy of IVDs obtained from cadavers following IDET, including extensive collagen disorganization, decreased quantity of collagen, collagen fibril shrinkage, and chondrocyte damage when compared with controls.19 Numerous theoretical explanations for these observations have been offered, such as changes in disc biomechanics, annular contraction, thermally induced healing response, sealing of annular tears, annular denervation, and decreased intradiscal pressure; each is briefly summarized here.
Changes in Disc Biomechanics
Although changes in the biomechanical properties of discs (e.g., compliance) could alter the function of a vertebral segment, studies have not provided clear evidence that this occurs. Studies in human cadaver discs after following IDET at 65° C to 90° C over 17 minutes with a 6-cm active tip thermal-resistive coil resulted in decreased disc compliance and increased motion in all planes, although this was greatest in lateral bending.20 The amount of increased motion did not, however, approach clinical significance and the authors concluded that IDET did not adversely affect segmental stability.
Similarly, Wang and colleagues heated bovine disc core samples consisting of the vertebrae and disc nucleus in a water bath and found no difference in either stiffness or failure strength.21 Failures occurred only in the midsubstance of the specimen. In contrast, Kleinstueck and colleagues, in a larger series of discs, found that heating cadaver IVDs with IDET placed 10 to 15 mm from the outer annulus resulted in a significant (6% to 12%) decrease in stiffness, though temperatures greater than 65° C were seen only within a 2-mm radius of the catheter.22 To achieve a more uniform heating, Bass and colleagues heated their human cadaver segments in a water bath to 75° C and found that the annulus was more compliant at higher applied stresses.23
Annular Contraction
A decrease in the size of disc herniations secondary to contraction of collagen in the annulus could potentially lead to decreased discogenic pain. Indeed, when bovine annular tissue was placed in a tube and heated, Schaufele and colleagues measured a 2% decrease in mass, 15% decrease in volume, and 3% decrease in diameter.24 In addition, there were two cases in which magnetic resonance imaging (MRI) showed a reduction in herniation size after clinical application of heat using a thermal-resistive coil. However, a retrospective case series by Cohen and colleagues reported new disc herniation in 2 of 79 (2.5%) patients following IDET.25 He postulated that the slow recovery post-IDET and observed results were more consistent with collagen modulation rather than reduced nociception. Additionally, Bass showed that the annulus will only shrink 8% after heating to 85° C, compared with 60% to 80% in the shoulder capsule. Further shrinkage is likely prevented by the attachment of the annulus to the vertebral end plates.
Thermally Induced Healing Response
Heating is thought to first disrupt the weaker intramolecular bonds unraveling and denaturing the triple helix. The stronger nonreducible intermolecular cross-links remain intact and thus the amount of collagen is unchanged. This “melting” into an amorphous state causes the observed “tissue shrinkage” and is a phase transition from a highly ordered crystalline structure to a random coil state. The effect plateaus at temperatures above 75°C, but higher temperatures eventually cause tissue destruction. Because temperatures above 45° C cause necrosis, cells are assumed to be destroyed after treatment. By 2 weeks, animal experiments show an enhanced healing response with the proliferation of cells and capillary sprouting. However, the treated ligaments were two to three times more susceptible to cyclic and static creep strains and in one study, two in eight treated ligaments experienced partial failure during creep testing.26
Although there is a loss of cell viability staining following IDET, discs maintained in cell culture conditions have demonstrated the ability to recover within 2 to 4 weeks.27 Studies have demonstrated thermal modification of collagen after IDET treatment, with changes in both histologic and scanning electron microscopy collagen morphology, consistent with other studies showing that shoulder capsular tissues regain biomechanical integrity after thermal treatment. In contrast, an in vitro ovine study by Freeman and colleagues found that IDET did not modulate the healing response in any way at various time intervals up to 12 weeks.28 Both treated and untreated discs showed similar degrees of degeneration, and the sheep discs in which an annular incision was made showed the most pronounced degenerative changes. At 12 weeks, there was pronounced granulation tissue accompanied by small nerves up to the outer three layers.
Bass and colleagues confirmed these findings by heating bovine cervical discs and harvesting up to 3 months after the procedure.29 There was no evidence that thermal treatment lead to increased stability, and no indication that high temperatures delivered to large regions of the disc had any discernable therapeutic benefit. Similarly, Derby and colleagues found that in clinical practice, higher temperatures and larger total heating doses during IDET with catheters placed in the outer annulus increased the duration of subsequent pain and led to less favorable outcomes after 8 months, though long-term outcomes at 16 months were unaffected by these differences in heating protocols.11
Sealing of Annular Tears
Although several authors have postulated that IDET might “seal” annular tears, thereby leading to decreased pain, there are no clinical data to support this hypothesis. In fact, Narvani and colleagues reported that in 6 of the 10 patients with discogenic pain, MRI confirmed that high-intensity zones in the outer posterior annulus were unchanged 6 months after IDET.30
Annular Denervation
Studies using a heating element placed in close proximity to the outer annulus and heated according to protocol will most likely achieve temperatures greater than 45° C within 5 to 9 mm from the catheter.10,29,31 Because most current techniques involve catheters placed in the outer annulus, denervation is theoretically possible. Bono and colleagues showed that a zone of potential denervation occurred at distances 12 to 14 mm from the catheter.32 Using an IDET catheter placed close to the area of pathology, Wright and Gatchel measured mean outer annular temperatures of 43.9° C ± 2.3° C and concluded that these temperatures were sufficient to coagulate nociceptors.31 Although a catheter placed within 5 mm of the outer annulus will achieve temperatures toxic to nociceptors within outer and middle annular fibers, it is unclear if this mechanism correlates with clinical recovery after IDET.32 Of particular interest is the duration of subsequent pain, which may last from several days to a week because of tissue trauma, followed by clinical improvement. However, few studies have reported the frequency and duration of post-IDET pain. Derby and colleagues8 showed that in a series of 32 patients, subsequent pain lasted an average of 5 days. If patients having no or only minimal (<1 week) subsequent pain achieved a significantly better outcome, these data would be consistent with the theory that intradiscal heating reduces pain by destroying nociceptor input.
Decreased Intradiscal Pressure
Using a recently introduced shorter electrothermal IDET catheter (SpineCATH intradiscal catheter, Smith & Nephew), the controlled intradiscal application of thermal energy on bovine disc material resulted in a 15% decrease in volume, 2% decrease in mass, and a 3% decrease in disc diameter.33 The authors postulated that this mechanism might provide an explanation for observed improvements in radicular symptoms in patients with lumbar herniated nucleus pulposus. In a similar study, Podhajsky and Belous found pressure reductions approximating 30% to 50% in sheep nucleus pulposus after IDET treatment using a 1.5-cm electrothermal device (decompression catheter, Smith & Nephew).33
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree