40 Intradiscal and Peridiscal Therapies for Discogenic and Radicular Pain
Intradiscal therapies are those that involve the placement under imaging guidance of a needle, probe, or similar device into an intervertebral disc with the goal to reduce the patient’s presenting symptoms. Chemonucleolysis, a term coined by Smith and Garvin in 1963 when they injected chymopapain into a disc for the treatment of sciatica,1 was the first intradiscal therapy; it was designed as a minimally invasive treatment for radicular pain caused by a disc prolapse. Chymopapain was eventually shown to be an extremely effective treatment, but ironically ceased to exist mainly for political reasons. However, the main thrust on epidemiologic grounds has been the search for a minimally invasive panacea for spinal origin pain, particularly nonspecific low back pain (NSLBP). As a consequence, numerous device companies have developed experimental intradiscal devices, some of which have achieved modest success in the small cohort of patients who present with NSLBP and are given the questionable diagnosis of discogenic pain (DP).
The diagnosis established by provocation discography (PD) is internal disc disruption (IDD). In IDD, the disc is shown to be internally disrupted and the probable source of pain, and it is thought that the radial fissures are the prime source of pain. The credibility of the diagnosis IDD is dependent on the validity and reliability of discography. The diagnostic confidence of IDD can be manipulated by altering the criteria for calling a PD test as positive. Indeed, over the years these criteria have changed and it may be that they will change in the future. Initially discography was deemed positive when internal disruption was seen, but when morphologic change alone was shown to be nonspecific, pain provocation was added. With the recognition that pain provocation was itself nonspecific, the nature of pain provocation was redefined so that pain had to (1) reach a minimum intensity (currently about 7/10 on a VAS); (2) be exactly like the clinical pain; and (3) not be produced at adjacent discs The Diagnostic Criteria for IDD from the International Spine Intervention Society (ISIS) are presented in Table 40-1.2 It may be that further refinements of discography will increase the confidence that IDD is a credible diagnosis. If it is, then diagnosis should lead to a predictable outcome for this cohort, be it with conservative or interventional management.
Table 40-1 Diagnostic Criteria for IDD from ISIS (2004)∗
| 1. Reproduction of the patient’s pain by stimulation of the affected disc |
| 2. This pain must have an intensity of at least 7 on a 10-point scale |
| 3. This pain must be reproduced at a low pressure of stimulation: 15 psi (1 kg cm2) |
| 4. Stimulation of adjacent disc(s) must not reproduce pain |
| 5. Postdiscography CT demonstrates a grade III or IV fissure |
IDD, internal disc disruption; ISIS, International Spine Intervention Society.
∗ All categories must be satisfied. These criteria are being modified.
(From International Spinal Intervention Society. Lumbar disc stimulation. In Bogduk N (ed): Practice Guidelines for Spinal Diagnostic and Treatment Procedures. International Spinal Intervention Society, San Francisco, 2004, pp 20-46.)
Another problem in this area of medicine is proving treatment efficacy. The future development of treatments for IDD is likely to be constrained at least because of the costs of running multiple randomized controlled double-blind trials (RCDBTs). The estimated cost of a previous intradiscal electrothermal study was estimated to be more than $1,000,000.3 An alternative is to use well designed, properly conducted observational studies as the benchmarks that establish whether a particular procedure is backed by a suitable level of evidence of efficacy.3 Such studies are particularly good in at least refuting ineffective procedures and practices.3 The recent publication of the first RCDBT on MB suggests that it is an extremely effective treatment for IDD.4 Other studies are underway to further assess this treatment.
Intradiscal Thermal Therapies for Discogenic Pain
Intradiscal therapies for DP, and more specifically for IDD, are not standard treatments. They are in development. Bogduk has enunciated the status of intradiscal procedures elegantly as follows:5
Types and Methods
Discogenic and radicular pain can be treated with thermal intradiscal procedures (TIPs) that typically use radiofrequency (RF) energy delivered into the disc by a locally placed probe or similar device aimed at altering disc structure, removing disc tissue, or denervating nerves. Other patented energy sources that can be used include thermal, electric current, microwave emission, ultrasound emission, radioactive emission, and laser. Although RF is the most commonly used energy source, ultrasound is at least as good.6 RF technology has been used since at least 1891 when D’Arsonval used alternating current to prevent any unwanted effect of neuromuscular stimulation during surgery. Subsequently, Harvey Gushing developed the technique of high-frequency current for electrocoagulation of blood vessels during surgery.7 It was subsequently shown that high frequency lesions in the RF range were reproducible and well defined.8 Since this time, the technology has advanced, and RF current is used in multiple arenas, including neurosurgery, dermatology, chronic pain, and in the treatment of cardiac arrhythmias. The three minimally invasive TIPs for the treatment of IDD are intradiscal electrothermal therapy (IDET), percutaneous intradiscal radiofrequency thermocoagulation (PIRFT), and intradiscal biacuplasty.
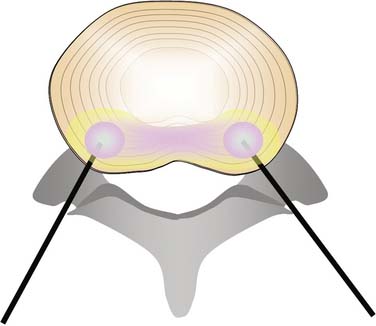
Intradiscal electrothermal annuloplasty (IDET) uses the Smith and Nephew Endoscopy (Andover, MA) SpineCath System in which a navigable catheter with an embedded thermal resistive coil is inserted posterolaterally into the AF. The catheter is then advanced through the disc circumferentially to return posteriorly. With the use of indirect radiofrequency energy, electrothermal heat is then generated with the thermal resistive coil; the disc material is heated for up to 20 minutes to 90° C (Fig. 40-1).
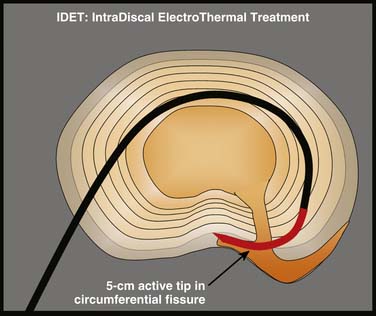
Figure 40-1 Schematic representation of IDET with the lesion seemingly applied across the radial tear.
Intradiscal biacuplasty (Baylis Medical, Montreal, Canada), uses RF energy directly to heat the AF while circulating water is used to cool the tissue that is adjacent to the needle. Two probes are placed on the posterolateral sides of the AF through introducer needles and the disc is heated by RF waves. The electrode surfaces are cooled by circulating water in the electrodes, thereby heating the disc tissue to 45 to 55° C—a temperature range that is considered to be neurotoxic.9–11 In fact, this technique can produce temperatures in the 46° to 67° C range in the inner two thirds of the AF, more than 45° C in the outer third and less than 42° C in the cauda equina,12 thus supporting other evidence that at least the procedure is safe13 (Fig. 40-2).
Heat Lesions: Extent and Effect
IDET and other thermal treatments were developed to produce intradiscal temperatures sufficient to at least ablate AF nerve fibers and also to denature AF collagen. RF lesions on peripheral nerves in dogs showed that at up to 6 weeks, there was a total loss of unmyelinated and a near total loss of myelinated fibers with lesions at temperatures as low as 45° C.14 This figure has not been verified in humans. It was considered that for denervation to occur, intradiscal lesions had to be above 45° C, but it may be that a significantly higher temperature is required. Safety was ensured by keeping temperatures in adjacent tissues below certain tolerance levels. Spinal cord and nerve root ischemia can be prevented by observing that the maximum tolerable temperatures in the CNS are 42.0 to 42.5° C for 40 to60 minutes and 43° C for 10 to 30 minutes.15 It had also been observed that collagen denaturation, from a triple helix to a random coil with variable crosslinking,16 occurred in other tissues at 60° -65° C.17,18 It was noted that this effect produced stiffening in shoulder capsule and tendons, leading to stabilization, as well as subsequent beneficial biologically mediated effects including increased strength; the process was accompanied by histologic changes, such as degradation and replacement of collagen fibrils, and signs of healing including increased cellularity, reactive fibroblasts, and increased vascularization.19 Thus, it was considered that thermal lesions in the disc could achieve collagen denaturation and denervation by producing intradiscal temperatures up to 65° C providing that surrounding structures were not heated beyond tolerance levels.
Subsequent cadaver experiments indicated that the temperatures developed by IDET could reach 60° C or more up to 2 mm from the catheter, 60° C between 2 mm and 4 mm away; and 45° C 9 mm to 14 mm away.20 It is notable that the region of increased temperature around IDET is small.16,20It was later reported that the temperatures reached in the AF by IDET were independent of the degree of DD,21 always reached 45° C, but did not always reach 60° C.21
Experimental Methods and Effects
Some of the cadaveric studies on thermal intradiscal thermal lesions have obvious limitations, not the least being their inability to model short- and long-term tissue responses to trauma.22–25 Live animal models also suffer because they address morphologic changes and not pain responses.
The most significant finding from these studies is that, perhaps consistent with morphologic differences between disc and shoulder capsule, the same process of stiffening does not occur in the in vivo animal disc;26–28 heat does not cause acute or chronic stiffening of the healthy disc.19,29 The first change that occurs after the introduction of any device within the disc is the development of fibrosis.30 There is no healing response.19 For example, at 12 weeks after disc incision, there is proteoglycan death, vascular granulation tissue consistent with an attempt at a healing response extending to one third of the AF depth, with grade 3 or 4 DD (Gries classification),31 and sparse neoinnervation in the outer AF. As an aside, it is notable that these changes do not necessarily occur with AF stab incision needles with a 19 gauge needle.21
The higher the temperature, the greater the AF destruction.21,26,32,33 Bass and colleagues performed an in vivo study, with animal sacrifice at 7, 45, or 180 days, on ovine cervical discs, demonstrating that high temperature treatment (70° C for 10 minutes) causes AF tissue degradation, but lower temperature lesions (52° to 54° C) have minimal adverse biomechanical effects compared to sham treatment.19 The most outstanding feature of intradiscal heat is it causes progressive deterioration of disc morphology without any healing response.19,21 Thus, the process is very different to that seen in joint capsule and tendon.
It is also unlikely that IDET lesions have an effect by denervation. It is theoretically possible that reinnervation of the NP is a significant mechanism for DP. Freemont and coworkers demonstrated a propensity for patients with IDD having fusion for LBP to develop reinnervation into the inner third of the AF (46%) and NP (22%), but also noted that these changes occurred but to a moderately lesser degree in nonpainful disc samples,34 a feature also noted in severely degenerated discs in any case.35 But do these thermal lesions actually denervate the disc? The heat required to produce denervation in peripheral nerves is at least 45° C.36 However, when IDET at 90° C was performed in sheep with experimentally induced posterolateral AF lesions with temperatures in the adjacent AF tissue raised to a mean of 63.6° C and in the NP of 67° C, there was not only a lack of tissue repair, but also a lack of denervation.21
Efficacy of Intradiscal Electrothermal Therapy
Intradiscal electrothermal therapy (IDET) is, by far, the most studied intradiscal therapy. Studies on IDET have been confined to the treatment of IDD. IDET has not been tested on other cohorts, such as patients with NSLBP who, for example, have DP diagnosed with nerve blocks or on patients with MRI-detected morphologic changes. It has also not been studied to any significant extent on other areas of the spine. As IDET targets fissures in the peripheral AF, it is logical that it should only be performed if the cause of pain is in the peripheral AF. The detection of annular fissures is one of a number of criteria for IDD.37
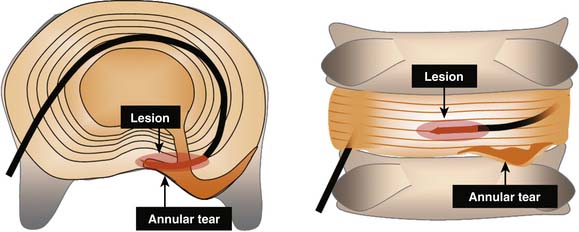
IDET is a technically demanding procedure. It requires precision: the lesion must be placed in the outer zone of the AF or across a radial fissure. IDET creates a small lesion (a few mm); even if the lesion seems well placed in axial views, it may be that in the horizontal dimension the lesion is applied above or below the painful fissure (Fig. 40-3).23 Additionally, if IDET works by neurotomy, the IDET lesion must be large enough to coagulate a critical number of nerve endings to produce pain relief:23 perhaps such widespread denervation is not achievable.
IDET has been found to, at best, benefit only a small cohort of patients with IDD and at worst to have no efficacy. The more important studies on IDET are on patients with IDD as determined by the strict ISIS criteria. In these studies, IDET has been performed optimally, with electrodes placed as far posteriorly in the AF as is feasible.23 Small lesions directed at poor targets should be no better than placebo!
The evidence about IDET derives primarily from two controlled trials on IDET using sham procedures as the control,38,39 and one prospective cohort study using patients denied interventional treatment as a comparison group.40,41 Although the Bogduk40,41and Pauza39 studies showed a number needed to treat (NNT) of five for excellent pain relief with the use of IDET, the Freeman42 study showed no benefit over placebo. Secondary information has been obtained from numerous observational case series.43–64 All of these studies, with the exception of one that did not record the device,46 used the SpineCATH. From these studies six systematic reviews have arisen.65–70
Group data from these observational studies show, in general, modest but definite improvements in pain and function,43,46,50,51,56,58,61,79, and these results seem to be much the same as might be expected from other interventional treatments including spinal fusion for the management of IDD. Reported outcomes have generally been the same for compensation versus noncompensation cases.40,41,47,80,81
There have been numerous systematic reviews that contain comments on IDET.65,66,70,84, Derby and associates consider that IDET provides a cheaper, less invasive, and safer alternative than does spinal surgery for the treatment of IDD—especially in patients with less functional impairment, relatively well maintained disc heights, and DP caused by annular tears or protrusions less that 3 to 4 mm.82 Derby further commented that of six IDET studies with sufficient reported data,39,40,46,52,85 between 38% and 94% (average 71%) of patients reported greater than 50% of pain relief 6 to 24 months after IDET.82
Overall, the success rates for IDET are modest. This may reflect the fact that IDET is a technically demanding procedure. It requires precision: the lesion must be placed in the outer zone of the AF or across a radial fissure, and even if on an optimal position, it creates only a small lesion (a few mm). Even if the lesion seems well placed in axial views, it may be that in the horizontal dimension the lesion is applied above or below the painful fissure.23 Additionally, if IDET works by neurotomy, the IDET lesion must be large enough to coagulate a critical number of nerve endings to produce pain relief:23 perhaps such widespread denervation is not achievable.
Randomized Controlled Studies on IDET
Pauza found 1360 eligible patients after a telephone interview, and, after performing discography on 260 of them, found 64 who met the criteria for IDD using pressure-controlled PD.39 Patients were treated with IDET or sham IDET, with the catheters in excellent position, and were followed for 6 months. The mean pretreatment VAS was 6.6 in the IDET group and 6.5 in the control group, and at 6 months, the improvement in VAS for IDET was 2.4 versus 1.1 in the control group. There was also a significant improvement of physical function in disabled patients and a trend to improvement in SF-36 Bodily Pain subscale. Approximately 40% of patients experienced greater than or equal to 50% pain relief, and approximately one out of five experienced greater than or equal to 75% pain relief.
Freeman performed a prospective, randomized, double-blind, placebo-controlled trial of IDET comparing this treatment group with a sham procedure for the treatment of chronic discogenic low back pain. Inclusion criteria included the presence of one- or two-level symptomatic disc degeneration with posterior or posterolateral annular tears as determined by provocative computed tomography (CT) discography.87 Of the 57 patients, 38 had IDET and 19 had sham IDET. The IDET catheter was positioned to cover at least 75% of the annular tear as defined by the CT discography. Follow-up was for 6 months. This study demonstrated no significant benefit over a 6-month posttreatment period for IDET. Interestingly, neither the sham nor the treatment group made any improvement over this period; there was no placebo effect.
Prospective Cohort Study on IDET
Bogduk and Karasek performed a prospective cohort study with 2-year follow-up in a group of 53 patients with similar pain scores evaluating the use of IDET after establishing the diagnosis of IDD according to ISIS protocol and then performing IDET on 36 patients and comparing them to another group of 17 patients who were refused treatment by the insurers.40 The comparison group can be considered representative of the natural history of LBP treated by conventional and conservative treatment regimens, although there may be a degree of negative effect relating to the intransigence of the insurers; perhaps this group then reflects the normal situation in real medicine because the insurers often are a significant barrier to patient management. This study found that the comparison group stayed the same in regard to pain over the 2 years, with only one individual reporting partial improvement, the rest no significant improvement, and none being completely relieved at either 12 or 24 months. This contrasted substantially with those treated with IDET; at 24 months, 54% of these patients had achieved at least 50% relief of their pain, were no longer using opioids, and were at work. Even more significantly, seven patients (20%) were totally free of pain and at work at 24 months. Thus, this data meant that for IDET, the number needed to NNT for a complete resolution of pain was five, and the NNT for 50% reduction in pain was three. Furthermore, 54% of IDET patients experienced a 50% or better reduction in pain and return to work and no need for opioids compared to 10% of the group undergoing typical treatment including rehabilitation.23
Efficacy of Percutaneous Intradiscal Radiofrequency Thermocoagulation (PIRFT)
Efficacy of Intranuclear PIRFT
In intranuclear PIRFT, a needle is inserted into the disc through which an electrode or flexible catheter is placed into the center of the NP of the nucleus and is slowly heated to 50 to 80° C for 90 to 360 seconds. The initial pilot study by Van Kleef and colleagues reported some success in a case series study of 39 consecutive patients with a minimum of 12 months LBP undergoing PIRFT with a 90-second 70° C lesion protocol. At 8 weeks 21/39 patients (54%), 7 of whom had had previous operation and 14 who had no operation, reported an adequate reduction in pain; at 16 months 16/39 patients (41%) reported reduction in pain.88
Barendse and coworkers performed an RCT on a cohort of NSLBP patients (n = 28) selected on the basis of positive (more than 50% relief) AD performed at L4-5 and L5-S1.89 Patients were randomized and after the RF probe was inserted underwent PIRFT with 90 second 70° C lesions (n = 11) or sham (n = 13). At follow-up there was no difference between the two groups. Either the selection process was inadequate (AD alone at two levels does not comply with the diagnostic protocol for IDD) and/or the treatment method itself was inadequate.
Ercelen and associates sought to determine whether the selection method or technique parameters made any difference to the RCT outcome.90 After 60 patients were diagnosed with IDD with PD, 39 patients were randomized into lesions over 120 seconds and 360 seconds both at 80° C. There was no difference between the groups at any time in the 6 months of evaluation. Pain scores were similar at 6 months to inception; the only difference was a significant decrease in pain in the first month seen in both groups. It could be concluded that PIRFT has no utility in the management of IDD or NSLBP diagnosed on the grounds of 50% relief with analgesic discography.
Azulay and coworkers used a different system to perform intranuclear RF thermal lesions on 17 patients with persistent IDD, based on at least one level positive PD, for at least 6 months (mean duration was 50.6 months).91 The criteria for diagnosing DP was abnormal T2-weighted images and if no radicular pain was present, disc bulging, or herniation. Treatment was not performed if the disc height was more than 50% reduced. The technique was different from previous descriptions; 0.9% saline solution was injected into the NP, impedance levels were monitored, and each disc took about 40 minutes to treat. It was considered that this treatment should deliver safe, persistent, comprehensive heat to the AF at a sufficient degree to cause neural ablation. Using 50% pain relief as a marker of success, 15 patients were responders at 1 month (88%), 9 at 3 months (53%), and 12 at 6 months (70.6%).
Efficacy of Annular PIRFT (Radiofrequency Annuloplasty with discTRODE)
There is one RCT study on RFA. As with intranuclear PIRFT, the RCT showed no benefit over placebo treatment.92 RFA has also been compared to IDET in trial with 42 patients matched into each treatment, with follow-up at 2 weeks and then at 2, 3, 6, 9, and 12 months; the IDET group had significantly better results from the 3-month period.62 In the IDET group VAS decreased from 7.4 ± 1.9 before IDET to 1.4 ±1.9 at 1 year follow-up, compared to a change from 6.6 ± 2.0 before to 4.4 ± 2.4 at 1 year after RFA.
Finch93 performed a case-controlled series on 46 patients with at least 6 months of LBP diagnosed as IDD at a single level. Thirty-one patients underwent RFA and the remaining group who could not get funding for the procedure persisted with usual care. After 12 months, the VAS decreased significantly by 37% in the RFA group, and did not change in the other group. Disability, as measured by the Oswestry Disability Index (ODI) also improved.
Subsequently, Kvarstein performed an RCT using RFA or sham RFA with the discTRODE probe with 10 patients in each group and followed the patients at 6 and 12 months.92 After selection according to strict criteria including positive PD with concordant pain at more than 7/10, patients underwent heating following the 10-minute protocol established by Finch,94 which is incremental heating starting at 50° C increasing by 5° C every second minute ending with a 4-minute interval at 65° C. There was no difference between the groups.
Efficacy of Disc Biacuplasty
Disc biacuplasty is in its infancy. Its safety has been studied by Kapural and colleagues,95 who found that it provided a more generalized heat throughout the posterior AF than did IDET, while not heating the CNS to excess. After a reporting successful outcomes on two patients,96 one of whom had previously had a surgical discectomy,97 Kapural and coworkers reported on a 6-month pilot trial on 15 patients with IDD diagnosed on PD—all with disc heights of more than 50% of controls.98 Reassessment occurred at 6 and 12 months.98,99 At 6 months, 13 patients were reassessed. The median VAS had reduced from 7 (±1) to 4 (±1) at 1 month, and stayed much the same at the 6-month and 12-month assessments. Disability and physical function scores also improved but the differences were insignificant. Opioid use continued to decrease after the 6-month follow-up.
Comments on Pulsed RF
Pulsed RF does not cause neural damage. A study on rat sciatic nerve compared the results of PRF lesions to that of RF lesions at 42° C and 70° C for 120 seconds. When the sciatic nerve was studied histologically at 21 days, it was recorded that most axonal damage occurred with the RF 70° C group.100 PRF does not cause significant damage to neural tissue,100 and any changes seem to be reversible.101 In egg white, PRF starts to produce minimal thermocoagulation at 60° C; above this temperature, PRF produces coagulation similar to, but smaller than, that of RF at the same temperature.102
PRF has been compared to RF treatment in the management of ZJ pain and been found to be substantially inferior. When 2 Hz PRF was compared to RF denervation at 80° C and to injection of local anesthetic for the treatment ZJ pain, with needles placed adjacent to the relevant medial branches or dorsal rami, there was a decrease in the VAS and disability scores at 6 and 12 months in the RF group but not the other two groups: PRF had a better short term outcome than did the control group but the improvement was not maintained at 6 or 12 months.103
Various studies have pilot-tested PRF. Intradiscal PRF has been pilot tested in a group of patients with IDD. It is theorized that high-voltage, long-duration intradiscal pulsed radiofrequency with the electrode in the center of the NP might work not from thermal effects but by exposure to electric fields.104 One pilot used PRF for 20 minutes at a setting of 2 × 20 ms/sec and 60 V on eight patients: it reported a reduction of pain score of 4/10 at 3 months and seven of eight patients were pain free at an average follow-up of 12.8 months.104 Pulsed RF has never been tested by an RCT and it seems that these results can be suitably ignored until an RCT on the efficacy is reported for any pain situation and then for the management for the management of DP or IDD.105
Neural RF Thermocoagulation for Discogenic Pain
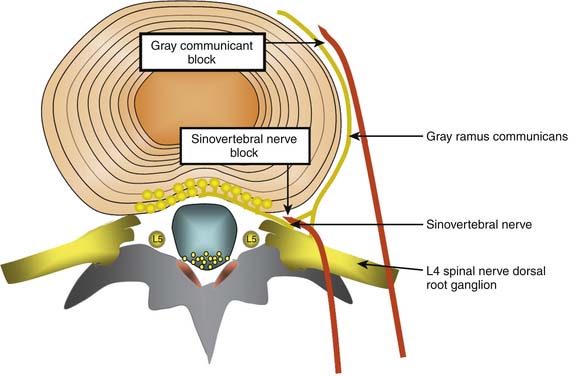
Conceptually, the possible minimally invasive targets for the treatment of a painful disc include the collagen and neural tissue within the disc, as well as the direct nerve supply of the disc (the sinuvertebral nerves, and the gray rami communicantes). As discussed earlier, it is difficult and perhaps impossible to selectively block the sinuvertebral nerve. The rami communicantes nerves are more accessible. However, both nerves innervate tissue other than the disc, so selective denervation of the disc is probably not possible in any case (Fig. 40-4).
Gray Rami communicantes
The efficacy of treatment to these nerves has not been established at any stage. Although RF of the gray rami communicantes was described in 1988 as being well tolerated and at times seemingly successful, it was noted that there was a discrepancy between clinical outcomes and the information gleaned by diagnostic blocks.106 Simopoulos and coworkers used L2 ramus communicans blocks as a basis for performing L2 ramus communicans RF lesions at 80° C for 60 seconds, and in a pilot study of five patients reported “consistent pain relief after a minimum of two radiofrequency lesioning treatments approximately 4 months apart.”107 However, there has been an RCT of RF treatment of these nerves that has established possible efficacy. Oh and Shim performed an RCT on a group of patients who had been diagnosed with IDD according to strict protocol and subsequently had failed treatment with IDET.108 Patients were then excluded if they had subsequent positive medial branch blocks for ZJ pain, and were included after they reported a 50% or greater improvement in pain following a diagnostic block of the appropriate ramus communicans with 2 mL 2% lidocaine and 1 mL contrast medium. The treatment group (n = 26) received RF thermocoagulation (Stryker system) of the ramus communicans nerve at 65° Cfor 60 seconds as well as local steroid injection; the control group (n = 23) received an injection of lidocaine without RF. Patients were followed only for an average of 4 months, and VAS scores were significantly lower in the treatment group at that time. In the treatment group VAS changed from 7.1 to 3.8 (± 1.1) and SF 36 subscales for bodily pain and physical functioning also improved significantly. There was no statistical change in the control group. This is an interesting study that needs to be replicated.
These nerves have also been injected in patients with refractory pain in association with osteoporotic vertebral compression fracture. In one retrospective study, gray rami communicantes blocks with local anesthetic and steroid at the fracture level produced or coincided with a modest improvement of pain over a 9-month period, with decreased analgesic requirements in 42% and medium-to-high patient satisfaction in 75%.109
Sinuvertebral Nerve Treatments
There is some reported evidence that the L2 nerve root might innervate the lower lumbar discs. If so, it might be possible to perform RF lesions to these nerves. However, it should be noted at the outset that bilateral L1 and L2 dorsal root ganglion (DRG) blocks with injectate including 4 mL bupivacaine 0.5% did not give any pain relief in 12 patients with IDD diagnosed with PD.110 As noted earlier, these nerves supply structures other than the disc, and any efficacy from sinuvertebral nerve treatment might have nothing to do with DP.
Murata and associates performed an RCT of L2 nerve root block on patients with NSLBP and/or radicular pain, reporting that the treatment group who had L2 spinal nerve root block with 1% lidocaine and 3.3 mg dexamethasone had a reduction of pain from 69/100 to 14/100 at 5 minutes and the control block group with the same medication at a depth of 2.5 cm had insignificant pain reduction from 68/100 before the injection to 62/100 5 minutes later; incidentally, they also reported a modest treatment effect at 1 and 10 weeks postinjection.111 They found that almost all patients with LBP or radicular pain had significant reduction of pain following the L2 nerve root injection, although it was not clear whether the leg pain was true radicular pain. Another study on the L2 nerve root was that of Nakamura and colleagues, who injected the L2 nerve root in 33 patients with NSLBP, some of whom had sciatica, and reported that needle insertion produced pain radiating to the low back in 23/33 injections, that LBP decreased substantially or totally, and that sciatica was not changed at all in the nine patients with sciatica.112
Furthermore, as sensory nerves from the L2 and L5 vertebral bodies may enter the paravertebral sympathetic trunks and reach the L2 DRG, Ohtori and colleagues performed an RCT on patients with LBP after acute L3 or L4 osteoporotic lumbar vertebral fracture. The treatment group had an L2 spinal nerve root block with 1.5 mL 1% lidocaine and the control group had a subcutaneous injection. Both groups had pain relief that was greater in the treatment group at 1 hour, 1 week, and 2 weeks after treatment, but there was no relief after this time in either group. It was concluded that the L2 DRG might innervate the L3 and L4 vertebral bodies in humans.113 In fact, this study randomized these patients into a treatment and a control group. The treatment group had injection of 1.5 mL 1% lidocaine directed onto the L2 nerve root with fluoroscopic control with contrast and the control group had the same injectate given subcutaneously. In the treatment group, VAS preblock was 70 ± 25 and VAS at 1 hour was 35 ± 23; in the control group, VAS preblock was 72 ± 22, and VAS at 1 hour was 56 ± 23. That is, although there was a possible trend for those patients with nerve root injection to have a decrease in pain, the confidence limits overlapped. It was not clear if any patient had 100% reduction in pain, a figure that might attract more interest if the L3 and L4 vertebral bodies are substantially innervated by the L2 DRG. Furthermore, Mendez and coworkers assessed the ability of anesthetic infiltration of the L2 spinal nerve to predict the results of PD. In 40 cases, L2 spinal nerve blocks were performed at least 2 weeks before PD. It was found that L2 nerve root block predicted the PD outcome in 46.5% of cases only, and the likelihood ratio of close to 1.0 determined that L2 nerve root block is not a surrogate for PD.114
Even if sinuvertebral nerve blocks eradicated pain and it was decided to treat with RF, the proximity of the sinuvertebral nerve to other structures—not the least being the spinal nerve root—makes such heating untenable. Sluijter has proposed the use of pulsed RF (PRF) as a nondestructive method of exposing tissue to RF electric fields.115 However, there is limited evidence that supports the use of low temperature lesions in any area of pain medicine. In a case series report, Tsou and associates performed PRF on the L2 DRG for patients with NSLBP without leg pain and added L3-S1 DRG for those with leg pain. The greatest improvement in pain occurred at 3 months, and at 3 and 12 months approximately 45% of patients had pain relief of 50% or more.116
Intradiscal Chemical Therapies for Discogenic Pain
Proliferants
In one study, 30 patients with chronic NSLBP for an average duration of 8.5 years were diagnosed with IDD on PD and treated with an intradiscal injection of a solution containing glucosamine and chondroitin sulfate combined with hypertonic dextrose and dimethyl sulfoxide (DMSO), which is an industrial solvent also used to treat interstitial cystitis. At an average posttreatment time of 12 months, Roland-Morris scores were 6.4±1.0 from 12.0±0.9 and VAS 3.0±0.4 from 6.1±. Overall, 17/30 (57%) did very well with average of 72% of disability scores and 76% of VAS, whereas the other 13/30 (43%) showed little or no improvement.117
The effect of intradiscal injection of a solution consisting of 1.5 mL 50% dextrose in water and 1.5 mL 0.25% bupivacaine was measured on patients with moderate-to-severe DD without herniation and with positive PD. Patients underwent biweekly injections (average number 3.5). At 18 months, 43% of patients had sustained improvement with an average reduction in VAS of 71%.118
In a pilot study, Derby and colleagues tested intradiscal injection of glucosamine and chondroitin sulfate combined with hypertonic dextrose and DMSO (N = 35) against IDET (N = 74) in patients with IDD diagnosed with PD.119 At 6 to 18 months the VAS improvement was 2.2 for the injection group and 1.3 for the IDET group, with 66% of the injection group and 47% of the IDET reporting feeling better.
Steroids
Intradiscal steroids have been reported for the treatment of disc problems including putative DP.120–125 They have been shown to be no more effective than placebo at 2 weeks and 1 year in patients with a positive PD.120,121 They might be effective in the short term for some patients with vertebral end-plate changes. The rationale for their use is that they interrupt the inflammatory pathway in their action as a phospholipase A2 inhibitor.
The first reports were by Feffer, who subsequently reported on 244 patients injected with intradiscal steroids finding that at follow-up between 4 and 10 years, 47% had recovered and the patients with predominantly LBP were more likely to recover.123,124
Graham compared chemonucleolysis with chymopapain to intradiscal hydrocortisone, with 20 patients in each group, in a double-blind study of patients with “discogenic back pain and sciatica.”126 The 2-year post-injection survey by an independent observer revealed that there was no statistical significance between the results, although the trend suggested superiority of CN, which had nine good, three fair, and eight unimproved results in 20 patients, contrasting with hydrocortisone, which produced three good, eight fair, and eight unimproved results in 20 patients—one patient dying from unrelated causes.
Then Wilkinson and Schuman performed 42 intradiscal injections on a series of 29 patients with NSLBP, and 18 injections on 13 patients with NSNP, none of whom had classic symptoms or signs of true radicular pain, although some were reported as likely to have radicular pain.127
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree