Fig. 15.1
Graphical representation of the Monro–Kellie doctrine . Any increase in a single component of intracranial volume (blood, cerebrospinal fluid [CSF], tissue) must be matched by a decrease in another if intracranial pressure (ICP) is to be maintained. When an intracranial mass or edema expands beyond the buffering capacity of venous blood and CSF, ICP will rise
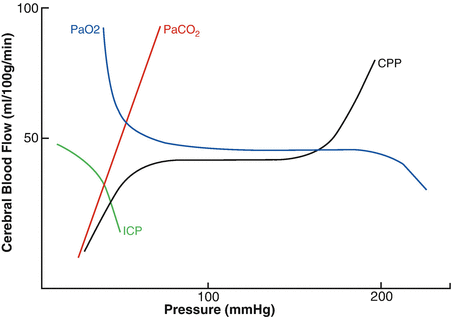
Fig. 15.2
Cerebral blood flow (CBF ) is maintained at a constant state when cerebral perfusion pressure (CPP) is between approximately 50 and 150 mmHg. This steady state of flow is provided by an intact autoregulation system. Above or below 50 and 150 mmHg, CBF is proportional to CPP. A PaO2 below 50 mmHg produces a significant increase in CBF. PaCO2 increases CBF by approximately 1 mL/100 g/min for every 1 mmHg increase in CO2. Increase in ICP can decrease CBF by decreasing CPP. CPP = MAP − ICP
The question of when to monitor ICP with an invasive device, as opposed to clinical presentation alone, has been a topic of vigorous debate. Numerous studies in recent decades have demonstrated that raised ICP in both adult and pediatric traumatic brain injury (TBI) is associated with poor outcomes [3]. ICP monitoring is also commonly utilized in patients with mass-occupying lesions, hydrocephalus, or cerebral edema , but formal guidelines exist only for patients with TBI. Whether monitoring ICP with a device can change outcomes while avoiding undue risk from the monitor itself is the real question. No randomized controlled trial exists to date that demonstrates a clear outcome benefit for patients with ICP monitoring and ICP monitor-guided therapy. Several cohorts and observational databases have demonstrated a trend toward improved outcome in high-risk populations , which prompted the Brain Trauma Foundation (BTF) to publish guidelines in 2007 for ICP monitoring in TBI based on the benefits seen in this trend [4]. The BTF recommends placement of an ICP monitor in all TBI patients who may be salvageable and who have an abnormal head CT, with the goal of using this information to optimize CPP. In addition, the BTF recommends establishing a guideline-based approach to treatment of raised ICP. Such a guideline-based approach, or protocol-driven management , has been shown to decrease both mortality and hospital costs while not increasing the numbers of disabled survivors. In recent years, many investigators have questioned the utility of ICP monitoring to guide patient management in subsets of high-risk patients such as the elderly [5]. A randomized trial of ICP monitoring vs. imaging and clinical examination-guided management in 2012 also found no outcome benefit of ICP monitoring [6]. Many have questioned its generalizability to TBI management in the developed world, while others suggest the study indicates that ICP monitoring is an important component of multimodal monitoring of perfusion after TBI [7]. The BTF guidelines continue to recommend ICP monitoring for guiding clinical management.
The intraventricular catheter (IVC ), often termed EVD for extraventricular drain, is the commonly held “gold standard” for ICP monitoring. The catheter measures global ICP , which is transmitted to the cerebrospinal fluid (CSF) filled ventricle and from there to fluid-filled tubing, which interfaces with a standard external transducer (Fig. 15.3). Alternatively, the catheter may contain a transducer within the lumen itself. Accurate interpretation of ICP by a ventricular catheter assumes an unobstructed connection between all CSF-containing compartments and free flow of fluid. The intraventricular catheter is introduced into the frontal horn of the lateral ventricle via a percutaneous route through a twist drill or burr hole made at Kocher’s point. Ideal positioning of the tip is in the ventricle, contralateral to the cerebral hemisphere with the disease-producing mass effect (Fig. 15.4). If the disease process leads to significant midline shift and effacement of the contralateral ventricle, then the catheter should be placed in the ventricle located within the hemisphere harboring disease to avoid worsening midline shift. The IVC transducer systems should be calibrated to a zero reference point at the foramen of Monroe, which conveniently coincides with the external auditory meatus. The IVC can be zeroed repeatedly after placement, which is in contrast to some intraparenchymal devices that cannot be recalibrated after insertion. The particular advantage of the IVC results from the option to use the ventricular catheter as a diagnostic and therapeutic tool. For example, in the event of malignant increases in ICP, the intraventricular catheter can be used to drain CSF, thereby decreasing pressure [8]. The dual utility has promoted the IVC to be the favored monitor in many neuroscience intensive care units. Additionally, the IVC can be used to administer pharmacotherapies such as antibiotics or thrombolytics as well as to provide for easy sampling of CSF for laboratory investigations.
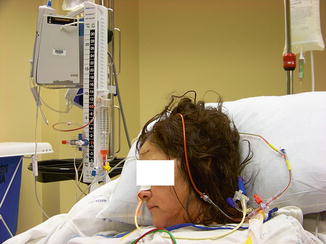
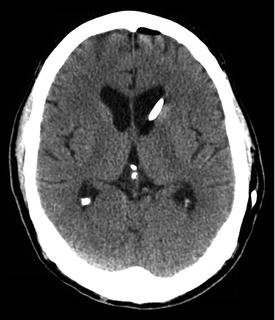

Fig. 15.3
An intraventricular drain placed in a patient following subarachnoid hemorrhage complicated by hydrocephalus

Fig. 15.4
Noncontrasted computed tomography (CT) of the head demonstrating correct placement of an IVC with the tip located in the lateral ventricle. Effacement of the ventricles is a relative contraindication to placement of an intraventricular catheter (IVC) or can be a sign of overdrainage of cerebrospinal fluid (CSF) when an IVC is already present. The IVC tip may also become entrapped in a collapsed ventricle, or displaced into the brain parenchyma, causing inaccurate measurement of ICP
Risks associated with placement of an IVC include bleeding and damage to eloquent tissues along the track toward the ventricle. In a coagulopathic patient, the risk of bleeding could produce high morbidity or even mortality and placement may be contraindicated. Multiple passes, in an attempt to place an IVC, can cause measurable tissue damage and only experienced providers should attempt to place this catheter. Risks also exist in transport or repositioning of a patient with an IVC. Unless a patient is in immediate danger of herniation, the IVC should remain clamped during transport or during significant patient repositioning to prevent inadvertent overdrainage of CSF and subsequent upward herniation (Fig. 15.5). It is best to secure the drain manifold to an IV pole. To prevent infection, the drain should never rest on the floor. Once the patient has been appropriately positioned, the catheter should be leveled to the external auditory meatus, unclamped and the IVC waveform transduced.
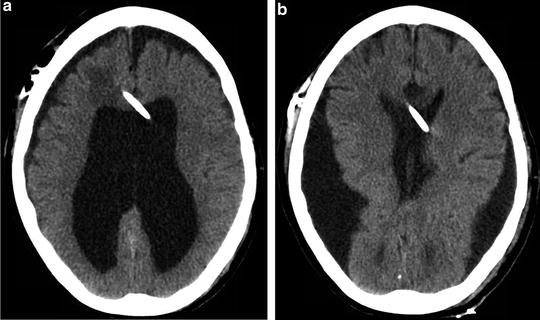

Fig. 15.5
(a) An intraventricular catheter (IVC) placed in a patient with a diagnosis of long-standing hydrocephalus . (b) The same IVC was left unclamped prior to transport. Neurologic deterioration was noted on examination and an emergent head CT demonstrated significant overdrainage of cerebrospinal fluid and ventricular collapse
Intracranial processes that lead to complete effacement of the ventricular system are considered contraindications to the IVC. In addition to the difficulty and risks with placement, information gathered from IVC monitoring in such scenario will be of limited value, as accurate ICP measurements via an IVC are dependent on the presence of CSF and unobstructed flow of fluid throughout the ventricular system to deliver accurate information. Risks associated with maintaining an IVC are largely centered on CSF infection. Catheters remaining longer than 5 days are at especially increased risk for infection; some studies report an incidence as high as 1–5 %. As with any device, care should be taken to maintain the highest level of sterility, utilize closed systems, and avoid any unnecessary sampling from the catheter. Compared with the intraparenchymal devices (discussed later), the IVC carries a much higher risk for infection [3, 9]. The added risk for the IVC comes from the column of fluid, which connects the hospital environment to the intraventricular system and provides a pathway for introduction of all manner of pathogens. In addition, the IVC system can be opened for sampling of fluid or administration of drugs , which incorporates risks that can be compared in principle with those of other indwelling catheters, e.g., central venous catheters.
Parenchymal devices to measure ICP can provide excellent diagnostic information and may be favored in clinical scenarios where an IVC is contraindicated. Several intraparenchymal devices are in use today. Each is placed directly into the brain tissue via a support bolt or tunneled subcutaneously before entering the brain via a burr hole. The Codman® microsensor (DePuy Synthes, Massachusetts, USA) is a miniature strain gauge that senses changes in pressure as an alteration in resistance across the tip. The Camino® (Integra LifeSciences Corporation, New Jersey, USA) is a fiber-optic device that senses pressure change as an alteration in a beam of transmitted light (Fig. 15.6). Both systems involve placement of a small system-specific probe into the brain parenchyma and with that carry similar risks and associated errors in interpretation. The risk of infection is much lower with intraparenchymal devices when compared with the IVC. Bleeding risks associated with the intraparenchymal devices may also be lower and therefore preferred when compared with the IVC in a patient with irreversible coagulopathy . Placement of the intraparenchymal device causes much less tissue damage and therefore less of a need for optimal coagulation physiology .
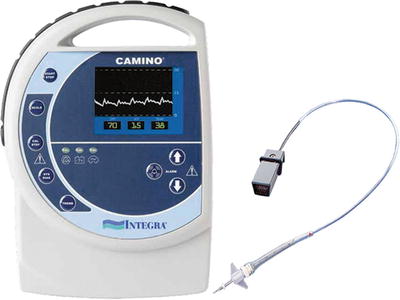

Fig. 15.6
Intraparenchymal fiber-optic monitor Camino® (Integra Lifescience, New Jersey, USA). This monitor is placed through a burr hole with the monitor tip in an intraparenchymal position
Information provided by both the IVC and parenchymal monitors is limited in that ICP is only measured in a relatively localized area of brain tissue [4]. Global ICP may not be reflected evenly throughout the cranium, particularly in the infratentorial vs. supratentorial cavities , as well as between right vs. left hemispheres (e.g., an IVC in the lateral horn may not accurately measure ICP in the posterior fossa). This type of variability has been well documented in studies comparing bilateral vs. unilateral cranial monitoring. Localized ICP provided by a space-occupying lesion may be physiologically devastating while causing little increase in global ICP such as the case in more subtle herniation syndromes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








