Key Clinical Questions
What are the types of intracranial hemorrhage, and how are they identified?
Which patients require emergent or nonemergent neurosurgical intervention?
How are aneurysmal and traumatic subarachnoid hemorrhage differentiated?
What medical treatments bear on the management of intracranial hemorrhage?
How is raised intracranial pressure managed?
Is anticoagulation safe after an intracranial hemorrhage?
How is brain death determined?
What tests should be obtained in patients with ventriculoperitoneal shunts and fever?
Introduction
Intracranial hemorrhages can be divided into three main categories. The first two, spontaneous intracerebral hemorrhage (bleeding within the brain parenchyma) and subarachnoid hemorrhage from aneurysm rupture, are considered strokes. The third category is intracranial hemorrhage from trauma, which may result in parenchymal, subarachnoid, subdural, or epidural bleeding. For ease of exposition, we use the term intracranial hemorrhage for all forms of bleeding within the skull and the term intracerebral hemorrhage for the more restricted category of bleeding within the substance of the brain.
When bleeding secondary to head trauma is excluded, intracerebral hemorrhage accounts for 10% of strokes. In the United States, rates range between 7 and 11 cases per 100,000 yearly, with a higher incidence in African Americans and Hispanics. The highest incidence globally is found in Japan, where rates are as high as 61 per 100,000 population.
Spontaneous Intracerebral Hemorrhage
The most common underlying condition in patients with spontaneous intracerebral hemorrhage is hypertension. Other associated conditions are coagulopathy, tumor, cerebral venous thrombosis, vascular malformation, amyloid angiopathy, and hemorrhagic transformation of ischemic stroke. The frequency of hypertension in patients with cerebral hemorrhage has been ˜75% in many population studies. Certain subgroups of hypertensive patients are at high risk for intracranial hemorrhage, including those younger than 55 years, smokers, and especially patients who have stopped taking chronically administered antihypertensive medications. Several prospective trials have demonstrated that the risk of cerebral hemorrhage decreases with administration of antihypertensive medications. Coagulopathy has become an increasingly frequent cause of primary intracerebral hemorrhage, with both medical anticoagulation and disorders such as uremia and liver failure contributing to this increase.
Several clinical features may help distinguish cerebral hemorrhage from other forms of stroke, such as headache, nausea, vomiting, confusion, systemic hypertension, and in advanced cases, decreased level of consciousness and enlargement of the pupils. The most common location for spontaneous intracerebral hemorrhage is the basal ganglia, accounting for ˜65% of cases. The presenting features, in addition to the above-described signs common to all hemorrhages, are hemiplegia and confusion. Thalamic hemorrhage presents similarly, usually with hemiplegia and often with hemianesthesia. Pontine hemorrhage causes rapid coma, quadriplegia, pinpoint pupils, and loss of extraocular movements. Cerebellar hemorrhage represents a special case that causes vomiting, vertigo, dizziness, gait ataxia, and progressive coma from hydrocephalus. Lobar hemorrhages are becoming more common with the prevalence of anticoagulant and antiplatelet therapy and cause signs referable to the region of bleeding. The differential diagnosis in all these cases includes ischemic stroke, and the distinction between ischemic stroke and hemorrhage must be made quickly.
Management of patients with intracranial hemorrhage can be separated into acute and subacute stages. Acutely, the greatest concern is for herniation, with secondary compression of the brainstem and irreversible brain damage. In addition, mass effect from a blood clot in the brain leads to elevated intracranial pressure, which in turn decreases cerebral blood flow and results in brain death. In the days following cerebral hemorrhage, a number of medical complications related to coma and immobilization become the focus of diagnosis and treatment.
Computed tomography (CT) scan without contrast is the primary diagnostic tool. CT angiogram or magnetic resonance imaging (MRI) may be required in special cases to establish the diagnosis of an underlying aneurysm, vascular malformations, or bleeding from an intracranial neoplasm.
All patients with suspected intracranial hemorrhage should have their coagulation parameters checked, specifically partial thromboplastin time (PTT), international normalized ratio (INR), and platelet count. Patients should be asked about anticoagulant medication, such as warfarin, aspirin, clopidogrel, and other antiplatelet agents, and recent cranial trauma.
The main considerations for the hospitalist are correction of coagulopathy, blood pressure management, and seizure prophylaxis. The management of elevated intracranial pressure (ICP) results in several predictable secondary medical problems, as discussed below.
Patients receiving chronic warfarin therapy require correction of the INR to a level < 1.3 to 1.4. This is typically accomplished with vitamin K 10 mg, preferably IV, and with infusion of fresh frozen plasma, while sequentially measuring INR. If emergency neurosurgical intervention or other surgery is required, concentrated recombinant factor VII or factor IX may be used, with the understanding that they may subsequently increase systemic clotting risk. In our experience, these factor compounds have been effective in reversing the effects of warfarin within 20 minutes. Patients receiving antiplatelet medications, probably including low-dose daily aspirin, should receive 6 units of platelets. In thrombocytopenic patients, it is probably advisable to correct the platelet level to > 100,000 if feasible. It has not been established if abnormal platelet function in patients with chronic renal failure requires platelet transfusion, but we often employ this strategy.
Most patients with cerebral hemorrhage are acutely hypertensive from the effect of raised ICP on barosensitive areas in the medulla. A reasonable approach is to keep mean arterial pressure between 70 and 100 mm Hg, which balances the risks of expansion of the clot from hypertension, and decreased cerebral perfusion from hypotension. Nicardipine and labetalol are frequently used antihypertensive drugs in neurologic practice, as they are short-acting and easily titrated. Patients who are chronically hypertensive may be maintained on their home medications. After the acute stage, β-blockers or angiotensin-converting enzyme inhibitors are generally used, but the choice is determined more by local practice and other medical factors.
|
In most centers, antiseizure drugs are administered for several weeks or until resolution of the clot, although this practice has not been studied in large randomized trials. The preferred anticonvulsant varies between institutions. We use levetiracetam, as opposed to phenytoin, given the more favorable side effect profile and predictable kinetics of the former. Phenytoin use has been associated with worse outcomes after intracerebral hemorrhage in some series.
There is evidence from one large and several small controlled trials that surgical evacuation of spontaneous intracerebral hemorrhage does not affect outcome. However, there may be exceptions depending on the clot’s location, size, and proximity to the cortical surface. Hydrocephalus an accompaniment of some hemorrhages, usually mandates intraventricular drainage or surgical evacuation of the clot. Several small series suggest that corticosteroids do not improve outcome.
Aneurysmal Subarachnoid Hemorrage
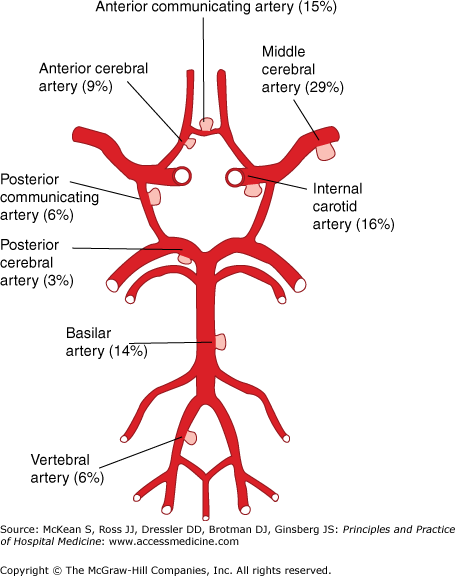
The typical presentation is a sudden, extreme headache described as “the worst of my life,” often associated with vomiting and various degrees of neck stiffness. Patients may have milder “sentinel” headaches in the days and weeks prior to presentation, perhaps as a result of small prodromal hemorrhages or aneurysmal stretch. Approximately 75% of subarachnoid hemorrhages are due to rupture of intracranial aneurysms (Figure 209-1), with another 10% attributed to arteriovenous malformations (AVMs). Subarachnoid hemorrhage due to AVM may cause less severe headache. Patients may be alert or suddenly become comatose. Systemic hypertension and meningismus are common on examination. The sudden rise in ICP in subarachnoid hemorrhage may produce intraocular bleeding, or Terson syndrome, which is associated with higher mortality. The clinical state after subarachnoid hemorrhage is graded by the Hunt and Hess scale (Table 209-1), sometimes referred to as the “H and H” score. Complications of subarachnoid hemorrhage may be severe and include acute and delayed hydrocephalus, vasospasm with resultant ischemic stroke, and rerupture of the aneurysm, which is often fatal.
| Grade | Description |
|---|---|
| I | Asymptomatic or with slight headache |
| II | Moderate to severe headache, nuchal rigidity; cranial nerve palsy |
| III | Confusion, drowsiness, and mild focal deficit |
| IV | Persistent stupor or semicoma, early decerebrate rigidity |
| V | Deep coma and decerebrate rigidity |
The best initial test is a noncontrast head CT scan, which has a sensitivity of 95% within 24 hours after aneurysm rupture but diminishes to < 75% by the third day. If the CT scan fails to demonstrate hemorrhage in a case with compatible clinical features, then a lumbar puncture should be performed to detect blood and blood products. The opening pressure is high (> 20 cm H2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree