(1)
Division of Pulmonary and Critical Care Medicine, Eastern Virginia Medical School, Norfolk, VA, USA
Keywords
Intracerebral hemorrhageSub-arachnoid hemorrhageCerebral vasospasmSubdural hematomaTranspulmonary thermodilutionTranscranial DopplerRaised intracerebral pressure (ICP)MannitolHypertonic salineIntracerebral Hemorrhage
Approximately 15 % of all strokes are hemorrhagic [1]. Intracerebral hemorrhage (ICH) is one of the most devastating forms of stroke. The mortality rate in the first 30 days after ICH is 40 % with more than half of the deaths occurring in the first 2 days; only 12–39 % of patients achieve functional independence [1]. The incidence of ICH increases with age and is more common in Asians than Caucasians or blacks. Clot volume at presentation is the most powerful predictor of outcome. Clot volume can be measured using computer algorithms available in some CT scanners or it can be approximated by the ellipsoid method [2]: ellipsoid volume = (AP × LAT × HT)/2. Generally a good functional outcome is associated with a hematoma volume of less than 30 mL [3–8]. Other important variables are clot expansion, patient age, baseline neurologic status, site of hemorrhage and intraventricular hemorrhage volume. Basal ganglia bleeds generally have the best prognosis followed by lobar hemorrhages; patients with pontine and brain stem bleeds have the worst prognosis [3, 6]. The most common sites of ICH are listed in Table 43.1. In patients who present within 3 h of symptom onset, 26 % of hematomas expand more than 33 % over the first hour, and another 12 % expand this amount over the next 20 h [9, 10]. In warfarin-associated ICH up to 50 % of patients develop hematoma expansion [11]. A number of prognostic scoring systems have been developed to risk stratify patients with ICH [5, 8, 12]. The modified ICH prognostic score and the Functional Risk Stratification Score (FUNC) are based on hematoma volume, age GCS, hematoma location intraventricular hemorrhage and pre-morbid cognitive status (see Table 43.2) [4, 7]. The STITCH investigators have developed a prognostic score based on the GCS at presentation, the patients’ age and hematoma volume: 10 × GCS − (age − 0.64 × volume). A score of 27.67 discotomizes patients into a poor and good prognostic group [13–15].
Table 43.1
Common sites for ICH
% | Location |
|---|---|
50 | Basal ganglia |
15 | thalamus |
10–15 | Pons |
10 | Cerebellum |
10–20 | Cerebral white matter |
1–6 | Brain stem |
Table 43.2
ICH prognostic score
Variable | Modified ICH score | FUNC score |
|---|---|---|
Hematoma volume (mL) | ||
<30 | 0 | 4 |
30–60 | 1 | 2 |
>60 | 1 | 0 |
Age (yr) | ||
<70 | 0 | 2 |
70–79 | 0 | 1 |
>79 | 1 | 0 |
Glasgow Coma Scale | ||
3–4 | 2 | 0 |
5–8 | 1 | 0 |
9–12 | 1 | 2 |
13–15 | 0 | 2 |
Hematoma location | ||
Lobar | 0 | 2 |
Deep | 0 | 1 |
Infratentorial | 1 | 0 |
Intraventricular hemorrhage | 1 | – |
Pre-ICH cognitive impairment | – | No = 1, Yes = 0 |
Risk factors for ICH include:
Hypertension
Age
Previous CVA
Moderate/heavy alcohol consumption
Amyloid angiopathy
Recreational drugs
Male
Oral anticoagulants
AVM’s
Hemorrhagic transformation of AIS especially post rt-TPA
Brain tumor
Venous thrombosis
Eclampsia
Post-partum cerebral angiopathy
Medical Management
Current practice (at least in the US) and clinical practice guidelines suggest that all patients with ICH be referred to a tertiary care center, be admitted to an ICU and undergo emergent neurosurgical evaluation [16]. However, as will be reviewed below, and with few exceptions, there is little data that neurosurgical interventions favorably alter patient outcomes. Patients with small (hematoma volume <15 mL) basal ganglia, thalamic and lobar hemorrhages without intraventricular involvement are unlikely to require a neurosurgical intervention. Likewise patients with catastrophic bleeds (hematoma volume >60 mL) are unlikely to benefit from any intervention. Patients with cerebellar bleeds are likely to require a neurosurgical intervention and therefore likely to benefit from transfer to a tertiary care center. While placement of a ventriculostomy is common in patients with intraventricular hemorrhage, it is not clear that this intervention improves functional outcomes (see below). Nevertheless, patients with IVH or those at greatest risk of developing this complication (hematoma volume >15 mL) [17] and who have a reasonable prognosis for functional recovery (hematoma volume <60 mL) are probably best managed at a tertiary care facility with the availability of neurosurgeons. Patients with ICH who have an elevated blood pressure (SBP > 150 Hg) at presentation will likely require a continuous infusion of an anti-hypertensive agent (see below); these patients require transfer to an intensive care unit. Similarly, patients taking an oral anticoagulant will require emergent reversal of the anticoagulant; this is best facilitated in an ICU. However, similar to the situation of patients with AIS, non-hypertensive patients with ICH and those unlikely to require a neurosurgical intervention can be adequately managed in a stroke unit. A proposed triage scheme is illustrated in Fig. 43.1 It should be emphasized that this triage scheme should only be used as a starting point and the patients age, neurological deficit, co-morbidities, pre-morbid functional status and advance directives should be used to develop an individualized treatment plan. For example, a 25 year old female with hemorrhagic conversion of a venous sinus thrombosis in a non-dominant hemisphere resulting in ICH >60 mL may benefit from aggressive supportive care in an ICU.
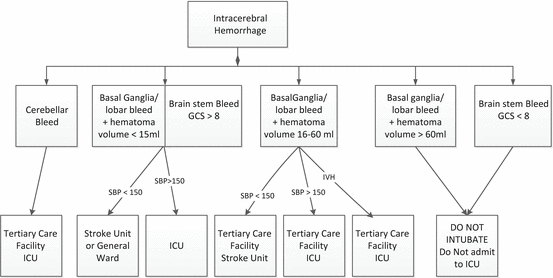

Fig. 43.1
Proposed triage scheme for intercerebral hemorrhage
The principles of the medical management of intracerebral hemorrhage are similar to those of acute ischemic stokes, with a few exceptions, notably blood pressure control. A NECT has a high sensitivity and specificity for the diagnosis of ICH. Common sites of bleed in patients with hypertension include the basal ganglia, thalamus and brainstem. Cerebral amyloid angiopathy is associated with lobar bleeds. Secondary causes of hemorrhage, including arteriovenous malformations, tumors, moyamoya, and cerebral vein thrombosis should be excluded in patients with unusual (noncircular) hematoma shape, the presence of severe edema, unusual location for hemorrhage, and the presence of other abnormal structures on imaging [16]. MRI/angiogram/venogram and CT angiogram/venogram are reasonably sensitive at identifying secondary causes of hemorrhage. An MR or CT venogram should be performed if hemorrhage location, relative edema volume, or abnormal signal in the cerebral sinuses on routine neuroimaging suggest cerebral vein thrombosis. A urine toxic screen should be obtained as part of the initial evaluation in ICH patients, particularly the young and the normotensive; substances implicated in the causation of ICH include cocaine, amphetamines, methylphenidate, talwin-pyribenzamine, phencyclidine and phenyl-propanolamine.
Patients undergoing treatment with oral anticoagulants constitute 12–14 % of patients with ICH, and with the increased use of these agents for the treatment of non-valvular atrial fibrillation and venous thromboembolism the proportion appears to be increasing. The risk of death is twice as high in those patients with ICH taking warfarin. In patients taking warfarin rapid reversal of the anticoagulant is recommended (see Chap. 38). Reversal of the newer oral anticoagulants is somewhat problematic. Emergent hemodialysis is suggested in patients taking dabigatran with residual anticoagulant activity (as evidenced by a prolonged PTT or ECT). In patients with ICH and mechanical heart valves temporary interruption of anticoagulation therapy seems safe in patients without previous evidence of systemic embolization. Discontinuation of anticoagulation for 1–2 weeks should be sufficient to observe the evolution of a parenchymal hematoma [16, 18]. Recommendations in patients with non-valvular atrial fibrillation is somewhat more complex. Studies of survivors of a first hemorrhagic stroke have identified rates of recurrent ICH of 2.1–3.7 % per patient-year, substantially higher than these individuals’ rate of subsequent ischemic stroke [19, 20]. The most consistently identified risk factor for recurrent ICH is lobar location of the initial ICH. Based on this information resumption of anticoagulation is not recommended in patients with a previous lobar ICH [11, 16]. In patients with a non-lobar hemorrhage and well controlled hypertension resumption of anticoagulation can be considered in those with a high risk of embolic stoke (based on the CHAD2 score) [11]. The effects of antiplatelet agents on ICH recurrence and severity appear to be substantially smaller than for anticoagulation, suggesting that antiplatelet treatment may be a safer alternative to anticoagulation after ICH [11].
Prior use of antiplatelet drugs is associated with an increased risk of death and worse outcomes following an ICH [21–23]. However, the role of platelet transfusion in patients taking anti-platelet drugs is controversial, with no clear benefit [22–24]. The AHA/ASA guidelines do not recommend routine platelet transfusion in these patients [16]. There is currently an ongoing study, the Platelet Transfusion in Cerebral Hemorrhage trial, which aims to answer this question [25]. A phase II study demonstrated an improvement in neurological outcome and mortality in patients with an ICH treated with activated recombinant factor VII (fVIIa) [26]. However, a large phase III trial (FAST) was unable to reproduce these findings [27]. Patients who are thrombocytopenic (platelet count <100,000) should be transfused with platelets.
Two randomized trials showed no benefit on regional blood flow, neurological improvement, mortality, and functional outcomes from the regular use of intravenous mannitol boluses [28, 29]. Patients with ICH are at an increased risk of DVT and PE. Prophylaxis with SCD’s is recommended in these patients. In immobile patients at increased risk of DVT, low dose s/c unfractionated heparin or LMWH can be considered if after 48 h there is no evidence of ongoing hematoma expansion [11, 16]. Prophylactic anticonvulsant medication are not recommended [16].
Blood Pressure Control
The acute hypertensive response in intracerebral hemorrhage is characterized by its high prevalence, self-limiting nature, and prognostic significance. In an analysis of 45,330 patients with intracerebral hemorrhage, 75 % had systolic blood pressure greater than 140 mmHg and 20 % greater than 180 mmHg at presentation [30]. The high blood pressure may be secondary to uncontrolled chronic hypertension, with disruption of central autonomic pathways by intracerebral hemorrhage. Alternately the high blood pressure may be an autoregulatory response to increased intracerebral pressure (ICP). High blood pressure is associated with hematoma enlargement and poor outcome [31, 32]. Systolic blood pressure reduction may reduce hematoma expansion in patients who are initially seen with an acute hypertensive response. However, reduction in blood pressure will reduce the cerebral perfusion pressure which may increase neuronal injury in the penumbral area. Until recently the risk/benefits of acute blood pressure reduction in ICH were unclear.
The Antihypertensive Treatment of Acute Cerebral Hemorrhage trial (ATACH I) was a pilot study that investigated the role of blood pressure reduction in 60 patients with ICH and a SBP > 170 mmHg [33]. SBP was reduced using intravenous nicardipine targeting three tiers of sequentially escalating SBP reduction goals (170–199, 140–149 or 110–140 mmHg). In this study hematoma expansion and poor 3-month outcome were greater in the group of patients having less aggressive BP reduction; due to the small sample size these difference were not statistically significant however aggressive BP lowering appeared to be safe.
The INTERACT2 trial randomized 2,839 patients with a spontaneous ICH within the previous 6 h and an elevated blood pressure to antihypertensive treatment to lower the SBP < 140 mmHg within one hour or a control group with a target SBP < 180 mmHg [34]. The primary outcome of death or major disability at 90 days did not differ between groups. However, an ordinal analysis showed significantly lower modified Rankin scores with intensive treatment. Subgroup analysis failed to demonstrate a group of patients who were harmed by intensive lowering of blood pressure. The use of antihypertensive agents was not standardized with urapidil (an alpha adrenergic blocker) being the most common agent prescribed (32.5 % of patients in the intensive blood pressure lowering group). Furthermore, drugs such as nitroglycerin, diuretics, nitroprusside and hydralazine which are “relatively contraindicated” in these patients (see Chap. 28) were prescribed in 45 % of patients in the intensive therapy group!!! In addition mannitol was prescribed in 62 % of patients. There were no significant absolute or relative change in hematoma growth in the intensive-treatment group as compared with the standard-treatment group; hence the reason for the apparent benefit of intensive blood pressure lowering in this study is unexplained. Despite the significant limitations of this study it would appear that lowering the SBP < 140 mmHg in patients with a spontaneous ICH is safe and may improve functional outcomes.
The ATACH II Trial is a large (n = 1,280) RCT designed to determine the efficacy of early, intensive, antihypertensive treatment (SBP < 140 mmHg compared to SBP < 180 mmHg) using intravenous nicardipine initiated within 3 h of onset of ICH and continued for the next 24 h in subjects with spontaneous supratentorial ICH [35]. The results of ATACH II should provide additional data on the safety and outcomes benefit of acute reduction of BP in hypertensive patients with an ICH. The SAMURI study demonstrated that in patients with ICH an infusion of nicardipine was highly effective in lowering the SBP < 160 mmHg with no obvious adverse effects [36]. ACCELERATE was a pilot study evaluating the role of clevidipine in patients with ICH and a SBP > 160 mmHg [37]. Clevidipine monotherapy was effective and safe for rapid BP reduction in this cohort of critically ill ICH patients. The median time to achieve SBP target range was 5.5 min Overall, patients showed minimal hematoma expansion with BP reduction, suggesting that rapid BP control with clevidipine may have a beneficial impact on hematoma expansion. Until the results of the ATTACH II study are available it would appear reasonable to lower SBP to <140 mmHg in patients with ICH and a SBP > 150 mmHg. However as discussed in Chap. 28, this should be achieved with the use of a continuous infusion of a short acting, titratable, intravenous antihypertensive agent (nicardipine or clevidipine only) in the controlled environment of an ICU.
Surgical Interventions
The STITCH trail randomized 1,033 patients with intracerebral hemorrhage to early surgery combined hematoma evacuation (within 24 h of randomization) or medical treatment [38]. Forty percent of hemorrhages were lobar, 40 % were in the basal ganglia/thalamus and about 20 % involved both sites with an average hematoma volume of 40 mL. Twenty-six percent of early surgery patients had a favorable outcome compared with 24 % randomized to initial conservative group (OR 0.89; 95 % CI 0.66–1.19). The mortality rate at 6 months for the early surgery group was 36 % compared with 37 % for the initial conservative treatment group (OR 0.95. 95 % CI 0.73–1.23). Patients with hematomas extending to within 1 cm of the cortical surface had a trend toward more favorable outcome with surgery within 96 h, although this finding did not reach statistical significance (OR; 0.69; 95 % CI 0.47–1.01). Patients with lobar hemorrhages and a GCS score of 9–12 also had a trend toward better outcome. By contrast, patients with ICH >1 cm from the cortical surface or with a GCS score of ≤8 tended to do worse with surgical removal as compared with medical management.
The STITCH II trial randomized 601 conscious patients with superficial lobar intracerebral hemorrhage (<1 cm from cortical surface of the brain) of 10–100 mL and no intraventricular hemorrhage to early surgery compared with an initial conservative treatment approach [14]. The primary outcome was a prognosis-based favorable or unfavorable outcome dichotomized from the Extended Glasgow Outcome Scale at 6 months. Seventy-five percent of patients had a GCS ≥ 12 on admission. Fifty-nine percent of patients in the early surgery group had an unfavorable outcome versus 62 % in the initial conservative treatment group (OR 0.86; 0.62–1.20). STITCH II enrolled patients most likely to benefit from clot evacuation and found no benefit. Based on the STITCH 1 and II trials there is therefore little data to support surgical evacuation of hematoma in patients with ICH. It should be noted that similar to almost all large multi-center studies, the patients in these trials were treated in neurosurgical centers of excellence. Such centers are more likely to have better outcomes than centers with lower surgical volumes and less expertise [39–41]. Therefore in the best of circumstances it would appear that surgical clot evacuation has little clinical benefit. It is likely that in centers with lower surgical volumes such surgery would be harmful.
Observational data strongly suggest that surgical decompression in patients’ with cerebellar hematomas greater than 3 cm in diameter or with brain stem compression improves outcome [42–44]. Due to the lack of clinical equipoise patients with cerebellar bleeds were excluded from the STITCH trials. The current AHA/ASA guidelines (which predate the publication of the STITCH II trial) states that “for most patients with ICH, the usefulness of surgery is uncertain but recommend surgery in patients with cerebellar hemorrhage who are deteriorating neurologically or who have brainstem compression”. Furthermore these guidelines recommend that “initial treatment of these patients with ventricular drainage alone rather than surgical evacuation is not recommended.” [16]
Intraventricular hemorrhage (IVH) is a frequent complication of ICH. In the STITCH trial, 23 % of patients had IVH on presentation of whom 55 % developed hydrocephalus [45]. In a series of 216 patients, Maas et al. reported that 21 % of patients with no IVH on presentation subsequently developed IVH [17]. In this study patients who developed a delayed IVH had a significantly greater clot volume than those who did not develop this complication. Furthermore, despite initially better clinical function compared with patients with initial IVH, patients with delayed IVH fared as poorly as the patients with an initial IVH in terms of mortality and severe disability. The severity of IVH varies from sedimentation of blood in the posterior horns to complete filling of all ventricles. Massive IVH is associated with a very poor outcome [46]. In the absence of any specific treatment, risks of death and severe disability in patients with IVH are reported to be 72 % and 86 %, respectively [47]. The risk of death and severe disability with placement of an external ventricular drain (EVD) is reported to be 58 % and 87 % respectively [47]. An EVD in the context of obstructive hydrocephalus would appears to decrease mortality compared to conservative treatment [46]. However, placement of an EVD does not improve the functional outcomes of the survivors. Placement of an EVD should therefore be considered in select patients with ICH who develop hydrocephalus with neurological deterioration. In patients with massive IVH, EVD may reduce mortality but result in severe functional disability. Intraventricular administration of rt-TPA is of uncertain benefit and is not routinely recommended [ 16]. The endoscopic retrieval of intraventricular blood has been recently described and appears to be to be as efficient as ventriculostomy, but its use is limited to specialized centers [46].
Subarachnoid Hemorrhage
Subarachnoid Hemorrhage (SAH) is a common and devastating condition. SAH accounts for about 5 % of all strokes and affects as many as 30,000 Americans each year [48]. Despite improved management the outcome following SAH remains poor; with an overall mortality of approximately 25 % and significant morbidity amongst the survivors [48]. The vast majority of patients with SAH have a ruptured aneurysm. In general the prognosis is related to the amount of blood in the subarachnoid space. SAH causes profound reductions in cerebral blood flow, reduced cerebral autoregulation, and acute cerebral ischemia [48]. These pathophysiological processes are linked to raised intracranial pressure, decreased cerebral perfusion pressure, vasoconstriction, platelet aggregation, with decreased microvascular perfusion and increased permeability [48, 49]. Despite advances in the understanding of the mechanisms of SAH-induced brain injury, few effective treatments exist. Furthermore, while numerous therapeutic interventions including putative neuroprotective agents been studied, few have demonstrated improved patient outcomes. Once the aneurysm has been secured treatment is essentially supportive. Scrupulous attention to the patients’ hemodynamic status may limit complications. As these patients are at risk of serious multisystem complications they are best managed in an ICU or a specialized neurology/neurosurgical unit.
The most serious complications following the initial bleed are rebleeding and cerebral vasospasm; management of patients with SAH is therefore is largely directed to avoiding these complications [48]. The risk of rebleeding (with conservative therapy) is highest in the first month, with a rate of between 20 and 30 %. The mortality rate is approximately 70 % for patients who rebleed. Angiographic vasospasm probably develops to some degree in most patients who suffer a SAH. However, clinically manifest vasospasm occurs in approximately 40 % of patients. Fifteen to 20 % of these patients will suffer a stroke or die despite aggressive management.
Diagnosis and Evaluation
CT scan and lumbar puncture. Non-contrast CT scanning is the diagnostic test of choice following a suspected SAH [48]. If the scan is performed within 24 h of the event, clot can be demonstrated in the subarachnoid space in approximately 90 % of patients. The diagnostic sensitivity of the CT scan declines after the first day. A diagnostic lumbar puncture should be performed in a patient with a suspected SAH if the initial CT scan is negative. A normal CT scan and a normal spinal fluid examination excludes a SAH and predicts a favorable prognosis in the setting of the sudden onset of a severe headache.
Clinical Classification. The Hunt and Hess Classification system is the most commonly used grading system to assess the severity of a SAH [48]. The Hunt and Hess grade has important therapeutic and prognostic implications.
I.
Asymptomatic or slight headache
II.
Moderate to severe headache, nuchal rigidity, no neurological deficit other than cranial nerve palsy
III.
Drowsiness, confusion or mild focal deficit
IV.
Stupor, moderate to severe hemiparesis
V.
Deep coma, decerebrate rigidity
Cerebral angiography. Selective catheter angiography is currently the standard for diagnosing cerebral aneurysms as the cause of SAH. Approximately 20–25 % of cerebral angiograms performed for SAH will not indicate a source of bleeding. It is generally recommended to repeat the angiogram in 2 weeks, because vascular spasm may have obscured the aneurysm. However, only a very small percentage of repeat angiograms will demonstrate an aneurysm. The risk of rebleeding in patients with normal angiograms is low; less than 4 % are reported to rebleed when followed for up to 10 years. CT angiography has improved to the point where some centers use it as the primary test to identify an aneurysm.
Initial Management
Bed rest with elevation of the head to 20–35o
Nimodopine has been shown in multiple RCT to significantly improve outcome after SAH, by reducing delayed cerebral ischemia and is considered the standard of care [50, 51]. Contrary to common belief, nimodopine does not appear to reduce the incidence of vasospasm [51]. The mechanisms by which nimodipine exerts its beneficial effects is not well understood but may involve neuronal as well as vascular factors. Nimodipine 60 mg q 4 per NG/oral should be started as soon as feasible. The dosage should be reduced (not stopped) in patients who develop hypotension [50].
Mild sedation/anxiolysis for grade I and II patients.
Ativan 0.5 mg q 4–6 PRN IV
Agitation/delirium:
Haloperidol (Haldol) 2.5–5 mg IV PRN and then q 2–4 as reqd. Check QTc interval
Quetiapine (Seroquel) 25–50 mg BID (oral)
Dexmedotomidine infusion
Pain
Tylenol Max 2,600 mg/day (oral, suspension, rectal)
Fentanyl 25–50 ug q 4 PRN IV
Morphine 2–4 mg q 4 PRN IV
Temperature/fever
Fever is common in patients with SAH (41–72 %) and is more common with poor-grade SAH patients who have more subarachnoid blood [52–54].
It is important to exclude infective causes of fever (blood cultures, CXR, BAL, PVL’s, CSF, etc.). Serum procalcitonin (PCT) has been shown to be useful in distinguishing between the systemic inflammatory response (SIRS) following SAH from infection [55]. In febrile patients a baseline PCT should be obtained and repeated every 2–3 days as indicated.
Fever should be treated aggressively and kept below 37.5 °C
Acetaminophen, Max 2,600 mg/day (oral, suspension, rectal)
Naproxen 250–750 BID or ibuprofen 300–800 mg TID (not contraindicated in patients with SAH) [50, 56].
In a prospective RCT the combination of ibuprofen and acetaminophen were shown to be more effective in reducing fever than either agent alone [56].
If fever not controlled with acetaminophen and a NSAID then active cooling with a cooling blanket/ external cooling device or an intra-vascular cooling system is recommended [57]. The patient may require anti-shivering medications (meperidine, dexmedetomidine).
Fluid management
Maintain euvolemia; on presentation most patients with SAH are volume depleted and require volume resuscitation. However patients with SAH have a very complex hemodynamic pattern that requires very careful hemodynamic management (see transpulmonary thermodilution hemodynamic assessment).
Careful fluid management is required to avoid hypovolemia which may increase the risk of delayed cerebral ischemia. However “hypervolemia” (volume overload) is also associated with worse neurological outcomes.
Despite being widely advocated, data supporting the use of prophylactic “hypervolemia” does NOT EXIST. Two RCT investigating the use of prophylactic hypervolemia demonstrated no benefit on CBF, vasospasm or patient outcome [58, 59]. However, the incidence of complications, primarily pulmonary edema was increased with “hypervolemia”. The concept hypervolemia is a misnomer and reflects a lack of understanding of cardiovascular physiology and the Frank-Starling mechanism (see Chap. 9).
0.9 % NaCl at 100 ml/h (adjusted according to cardiac, pulmonary, renal and electrolyte status)
Sodium
Keep Sodium between 140 and 145 meq/L.
Na < 135—check serum and urine electrolytes (including uric acid) and osmolarity. Calculate fractional excretion of urate (see Chap. 40).
SIADH—one dose of 20 mg conivaptan and follow Na (ensure patient not hypovolemic); repeat conivaptan as required [60].
Cerebral salt wasting—0.9 % NaCl. Salt tablets although commonly prescribed are probably useless. 3 % hypertonic saline can also be considered however the sodium should be closely monitored (see below).
Patients with SIADH have hyponatremia with features of water overload (hemodilution) while patients with cerebral salt wasting have hyponatremia with evidence of hemoconcentration. Transpulmonary thermodilution with measurement of the global end-diastolic volume (GEDVI) may help in the determination of volume status. Alternatively, the sodium can be corrected with an infusion of 3 % saline at 50–100 mL/h (the serum sodium should be checked after 2 h and then 2 hourly until the sodium has stabilized) with re-calculation of the fractional excretion of urate once the sodium has corrected (see Chap. 40).
Diabetes insipidus; replace with 0.45 NaCl and administer desmopressin 2 mg SQ bid
Magnesium
Magnesium has putative neuroprotective properties. The Magnesium for Aneurysmal Subarachnoid Hemorrhage (MASH-2) study did not demonstrate an outcome benefit from intravenous magnesium (64 mmol/day) [61]. While hypermagnesemia does not appear to be beneficial hypomagnesemia should be avoided to limit the risk of arrhythmias and other possible complications. As per usual ICU practice the Mg level should be kept >2 mg/dL.
Glucose control
Hyperglycemia is common following SAH [62, 63]. Hyperglycemia is associated with poorer clinical grade and worse prognosis [62, 63]. However, in keeping with ICU patients in general hyperglycemia is a reflection of the degree of activation of the stress response and DOES NOT imply a causal relationship between hyperglycemia and poor outcome (see Chap. 13).
A study of SAH patients treated with an insulin infusion targeted to keep the blood glucose between 80 and 110 mg/dL (tight glycemic control) found an increase in episodes of hypoglycemia, and this was associated with more vasospasm and a less favorable 3-month outcome [64].
Microdialysis findings in patients with SAH have demonstrated cerebral metabolic crisis and low cerebral glucose in patients treated with insulin infusions, even in the absence of systemic hypoglycemia [65, 66].
Keep glucose between 140 and 200 mg/dL in keeping with general ICU practice. Tight glycemic control is likely to increase the risk of poor outcomes.
NPH and Sliding scale insulin is recommended for glycemic control. Insulin infusions should only for used for severe hyperglycemia. Bolus feeding with a low glycemic index formula is likely to reduce the degree of hyperglycemia (see Chap. 32).
Corticosteroids have no proven benefit in patients with SAH, are likely to increase the risk of hyperglycemia (and myopathy) and should NEVER be prescribed.
Blood pressure control
Stop home (PO) anti-hypertensive medications and diuretics (not Beta blockers)
The role of anti-hypertensive agents in preventing rebleeding is controversial.
It is generally advised that the SBP be kept below 150 mg/Hg prior to securing the aneurysm. In this situation Nicardipine. Clevidipine or labetalol infusions are the preferred agents (see Chap. 28).
Mild sedation and control of pain may adequately control an elevated blood pressure
Exclude vasospasm in patients with increasing BP.
An increase in BP may be a manifestation of an increased ICP. Lowering of the blood pressure in this circumstance may have serious adverse sequela. Such therapy should only be considered in patients where the ICP and CPP are being monitored. In such situations Nicardipine. Clevidipine or labetalol infusions are the preferred agents.
Antihypertensive agents should be used with great caution as an excessive reduction of blood pressure may cause cerebral ischemia and infarction.
Laxatives (Bisacodyl, lactulose)
Anemia
DVT prophylaxis
SAH induces a prothrombotic state that may lead to an increased risk of DVT and PE [50].
Sequential compression devices are recommended on admission.
Prophylactic heparin 5,000 u s/c q 12, LMWH or fondaparinux are recommended 48–72 h after coiling/craniotomy [50].
Consider screening PVL’s every 4–5 days
Mechanical ventilation
Sedation with propofol is recommended (keep dose <50 ug/kg/min). Fentanyl may be added as needed. Dexmedetomidine is an alternative agent.
Keep PaCO2 35–40 mmHg
Keep arterial saturation between 92 and 96 %
Keep plateau pressures <30 cmH2O
Monitor iPEEP; keep <5 cmH2O
Nutrition
Start within 24 h.
Bolus tube feeds (see Chap. 32).
Statins. Preliminary data suggested that statins improved the outcome after SAH. However, recent meta-analyses have failed to demonstrate a benefit in terms of the risk of vasospasm, delayed cerebral ischemia, poor outcome or mortality [69, 70]. Based on this data statins are not routinely recommended, however, a statin should not be stopped in patients already taking these agents (increases the risk for developing sepsis).
Stress ulcer prophylaxis: SUP is not required if patients are receiving enteral nutrition or taking a normal diet (see Chap. 33).
Transcranial Doppler’s (TCD’s): Daily TCD’s are recommended starting on the 3rd ICU day and continued for 7–10 day or as indicated. A CT angiogram’s is suggested if flow velocities are increasing (see below).
Brain tissue oxygen monitoring and cerebral microdialysis have been described in a number of observational studies [71–77]. While a brain tissue oxygen tension <20 mmHg, a lactate/pyruvate ratio >40 and a brain glucose concentration <13 mg/dL are associated with poor outcomes it is not clear that these monitoring techniques result in improved patient outcomes.
Screening ECHO (to assess LV function) and ECG (to exclude myocardial ischemia) on admission
The routine use of phenytoin has been associated with cognitive impairment and is not recommended [78]. Keppra is the preferred agent when the patient deemed at high risk of seizures
Corticosteroids have no proven benefit in SAH and are not recommended.
Specific Therapeutic Issues
Antifibrinolytic Therapy
The role of antifibrinolytic agent in preventing rebleeding is controversial. While antifibrinolytic agents reduce the rate of bleeding the benefits are offset by a higher incidence of cerebral infarction [79]. A more recent RCT (n = 505) demonstrated that an early short course of antifibrinolytic therapy reduced the risk of early rebleeding pending repair of the aneurysm [80]. Delayed and prolonged therapy with these agents is not recommended [50]. While a short course of therapy prior to securing the aneurysm may be beneficial these agents have generally fallen out of favor and are not widely recommended [50].
Surgical and Endovascular Methods of Treatment
In 1991, Guglielmi et al. described the technique of occluding aneurysms from an endovascular approach with electrolytically detachable platinum coils (Guglielmi detachable coils) [81]. Guglielmi detachable coils are introduced directly into the aneurysm through a microcatheter and detached from a stainless steel microguidewire by an electric current. The aneurysm is packed with several coils. The coils induce thrombosis, thereby excluding the aneurysm from the circulation. As clinical experience with the technique has increased and technological advances in coil design and adjunctive methods have improved, endovascular treatment has been used with increasing frequency [48].
The ISAT trial compared neurosurgical clipping versus endovascular coiling in 2,143 patients with ruptured aneurysms [82]. In this study which enrolled patients with ruptured intracranial aneurysms suitable for both treatments, endovascular coiling was more likely to result in independent survival at 1 year than neurosurgical clipping; the survival benefit continued for at least 7 years. The risk of epilepsy was substantially lower in patients allocated to endovascular treatment, but the risk of late rebleeding was higher.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





