Intracardiac Masses and Arterial Embolism
Carlos A. Roldan
The history and physical exam are of important value in the evaluation of patients with suspected cardiac substrates for embolism, intracardiac masses, or arterial embolism.
Although a negative history and physical examination predict a low yield of cardiac imaging for detecting cardiac sources of embolism, possible cardiac or vascular substrates of emboli such as patent foramen ovale, atrial septal aneurysm, valve strands, spontaneous echocardiographic contrast, aortic atheroma, and cardiac masses are asymptomatic and usually have negative physical findings.
The integration of the history and physical examination with cardiovascular and brain imaging are essential in establishing the diagnosis, mechanism, and management of patients with suspected intracardiac or aortic masses and arterial embolism (1).
Therefore, two-dimensional (2D) and real-time three-dimensional (RT3D) transthoracic (TTE) and in selected cases transesophageal (TEE) echocardiography (echo) are currently the most commonly used techniques for the detection of cardiovascular substrates for emboli formation, identifying and characterizing cardiovascular masses (thrombi, vegetations, atheroma, and tumors), and best guiding therapy in patients with arterial embolism (2,3,4).
Definitions
Cardiovascular Substrates for Emboli Formation
A cardiac or vascular pathology that predisposes to the formation of emboli and is associated with arterial embolism. Atrial fibrillation or flutter, myocardial infarction, left ventricular (LV) aneurysm, cardiomyopathy, infective and noninfective endocarditis, rheumatic mitral valve stenosis, prosthetic heart valves, and aortic atheromatous disease are the most common cardiovascular substrates for emboli formation. Cardiac tumors are uncommon substrates for emboli formation.
Intracardiac or Vascular Masses
Abnormal cardiac or vascular structures of variable size, appearance, shape, echoreflectance, and mobility. They are more commonly located within the cardiac chambers and are attached to the atrial or ventricular endocardium including the interatrial or interventricular septum, to the valve leaflets or annulus, or to the aorta. Rarely, they can be seen as freely moving masses.
Most common intracardiac and vascular masses include thrombus (Figs. 16.1 to 16.6), infective vegetations (Fig. 16.7), and aortic atheromas (Fig 16.8).
Uncommon intracardiac masses include noninfective inflammatory or thrombotic vegetations and malignant or benign cardiac tumors.
Common normal anatomic variants that may mimic intracardiac masses include LV bridging trabeculations, left upper pulmonary vein ridge, atrial appendage pectinate muscles, nodes of Aranti, moderator band, Eustachian valve, and Chiari’s network.
Arterial Embolism
Partial or complete occlusion of a cerebral or peripheral artery by an emboli originating from the carotid arteries, aorta, or left heart (5,6,7).
Paradoxical embolism is a rare cause of arterial embolism and is caused by an emboli that originates in the venous system or rarely from a right heart chamber and gains access to the arterial system via an intracardiac shunt or rarely a pulmonary arteriovenous malformation (8,9). The diagnosis requires four clinical criteria:
Venous thrombosis
Communication between right and left circulations
Right-to-left atrial pressure gradient during the entire or a portion of the cardiac cycle
Arterial embolism in the absence of common cardioembolic substrates
The diagnosis is definite when a thrombus lodged across an intracardiac shunt is demonstrated.
Pulmonary embolism is detected in approximately 60% of these patients and deep venous thrombosis in approximately 40%.
A patent foramen ovale (PFO) is present in 72% of patients, atrial septal defect (ASD) in 12%, pulmonary arteriovenous fistula in 12%, and ventricular septal defect in 4%.
Arterial embolism results in stroke or transient ischemic attack (TIA) in 80% of patients, ischemic limb in 15%, or visceral ischemia or infarct in ≤5% of patients (5,6,7). In patients with paradoxical embolism, emboli to the lower extremities occur in 50% of cases; to the brain in 35% to 40%; and to the coronary, renal, splenic, retinal, or mesenteric arteries in 10% to 15% of cases (8,9).
Stroke is an episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, with evidence of infarction of the central nervous system tissue.
TIA is a transient episode of neurological dysfunction of <24 hours duration caused by focal brain, spinal cord, or retinal ischemia, but without acute infarction.
Carotid atherothrombosis or carotid or aortic atheroembolism is the cause of ischemic stroke or TIA in approximately 40% of cases.
Lacunar or small-vessel disease is the cause in 15% to 20% of cases.
Cryptogenic or no identifiable cause despite a thorough evaluation constitutes approximately 25% of cases.
An undefined proportion of these cases may have a cardioembolic cause.
Cardioembolism is the cause in 15% of cases.
Coagulopathy, vasculitis, arterial dissection, migraine, or sickle cell disease is the cause in <5% of cases.
Epidemiology of Stroke and TIA
Incidence
Every year, about 795,000 Americans develop new (77%) or recurrent (23%) stroke (10).
The incidence rates of stroke per 1,000 person-years varies according to age as follows:
2.4 for white men and women 45 to 54 years of age
6.1 for white men and 4.8 for white women 55 to 64 years of age
12.2 for white men and 9.8 for white women 65 to 74 years of age.
These rates are 4% to 7% higher for black men and women.
The incidence of TIA is estimated to be 200,000 to 500,000 per year or 68 to 83 per 100,000 individuals (higher in black men and in those >85 years old) (10).
The 90-day risk of stroke after a TIA is approximately 10% (range, 3% to 17%) with 25% to 50% of events occurring within the first 2 days.
Prevalence
The prevalence of stroke is 2.7% in men and 2.5% in women ≥18 year old (10).
These rates are highest in non-Hispanic blacks (3.7% to 4%).
Also, prevalence rates increase from 2% for age 50 to 59 years to 12% for age ≥80 years.
The prevalence of TIA is 2.3%—1% higher for men and highest rates for those >65 years old.
In patients with stroke, the prevalence of prior TIA is approximately 15% (range, 7% to 40%) with almost half of them occurring during the preceding 7 days.
One-third of presumed TIAs are strokes based on evidence of cerebral infarctions on diffusion-weighted magnetic resonance imaging (MRI).
Echocardiography
As previously stated, 15% to 20% of cases of arterial embolism are cardioembolic in origin, and an undefined proportion of the 25% of patients with presumed cryptogenic stroke or TIA may have a cardioembolic pathogenesis (5,6,7,8,9,10,11).
Therefore, echo is essential in the workup and management of patients with suspected arterial embolism.
The American Society of Echocardiography has recently published a limited number of appropriate criteria for the use of echo in patients with suspected cardioembolism (2,3,4,11,12) (Table 16.1). However, these criteria are based on limited evidence-based data and may not apply to all patients. Therefore, echo (TTE and/or TEE) should be used to fit best a specific clinical scenario in an individual patient.
The highest diagnostic and added therapeutic benefit of echo, in particular of TEE, appears to be in younger patients without known cardiovascular disease and in older patients with cardiovascular disease, but not a well-defined indication for anticoagulation.
When integrated with the history and physical examination, the information obtained from echo help to better define a clinical syndrome as cardioembolic in at least one-third of patients and consequently better guide the need of anticoagulation or antiplatelet therapy, further workup, or other interventions (11).
Table 16.1 Class I or appropriate (score 7–9) indications for echocardiography in patients with suspected arterial embolism
In patients with suspected or evidence of transient ischemic attack, stroke, or peripheral embolism and suspected cardiovascular source of embolus (TTE/TEE)
In patients with suspected cardiac or aortic mass (TTE/TEE)
Use of TEE in a patient with a suspected cardiac mass or cardioembolism with a nondiagnostic TTE due to patient characteristics or inadequate visualization of relevant structures
Re-evaluation of prior TEE finding for interval change (e.g., resolution of thrombus after anticoagulation, resolution of vegetation after antibiotic therapy) when a change in therapy is anticipated (TEE)
Evaluation to facilitate clinical decision making with regards to anticoagulation, cardioversion, and/or radiofrequency ablation (TEE)
(Adapted from Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Coll Cardiol. 2011;57:1126–1166).
The current use of contrast agents that enhance LV endocardial border definition and RT3D echo has significantly contributed to the increased and accurate detection of cardiac substrates of emboli and characterization of intracardiac and vascular masses (3,11,12).
Also, the use of transcranial Doppler (TCD) echo for detection of microembolism plays an important role in establishing the pathogenic link between a cardiovascular substrate of emboli or an intracardiac or vascular mass and a clinical syndrome of arterial embolism (13).
Cardiac and Aortic Substrates for Emboli Formation
Atrial Fibrillation
The reported prevalence rates of atrial thrombi in atrial fibrillation of >2 days or of unknown duration range from 10% to 20% (14,15).
Rates of atrial thrombi are higher (14% to 40%) among patients presenting with a recent or acute arterial embolism, other concomitant cardiac sources of emboli, rheumatic mitral valve stenosis, LV systolic dysfunction (LVEF <40%), spontaneous echo contrast, low (≤20 cm/second) emptying atrial velocities, or aortic plaques (16,17,18,19).
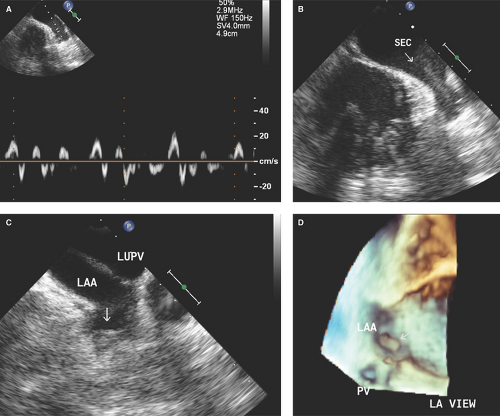
More than 90% of atrial thrombi in patients with atrial fibrillation are formed in the left atrial (LA) appendage (Fig. 16-1).
Atrial fibrillation causes 15% to 20% of all ischemic strokes, but >50% of all cardioembolic stroke/TIAs.
The risk of stroke/TIA in paroxysmal, persistent, or permanent atrial fibrillation or atrial flutter is probably similar (18).
The risk of stroke or TIA per year in patients with nonvalvular atrial fibrillation is currently based on the CHADS2 score, which assigns 1 point each for recent congestive heart failure (C), hypertension (H), age ≥75 years (A), and diabetes mellitus (D), and 2 for prior stroke (S) or TIA (14).
Score 0 = low risk or 1.2% stroke rate per 100 patient-years
Score 1 = low-moderate risk or 2.8 stroke rate per 100 patient-years
Score 2 = moderate risk or 3.6 stroke rate per 100 patient-years
Score 3 = high risk or 6.4 stroke rate per 100 patient-years
Score 4–6 = very high risk or 8–44 stroke rate per 100 patient-years.
Patients with a score of 0–1 are usually treated with aspirin and those with a score of 2–6 are treated with warfarin.
Myocardial Infarction
The risk of arterial embolism in patients with myocardial infarction (MI) is higher if the MI is associated with extensive wall motion abnormalities, LV aneurysm, moderate to severe LV systolic dysfunction (risk increases by 18% for every 5% decrease in LVEF), or atrial fibrillation (20). Therefore, the risk is usually higher in patients with anterior MI.
Patients with large anterior MI have an estimated incidence of LV thrombus of up to 20% (most formed within the first week) and an arterial embolism rate of 5% to 27% per year without anticoagulation.
Also patients with MI who have an LVEF <30% have a 1.86 risk of stroke as compared to those with an LVEF >35%.
Left Ventricular Aneurysm
LV aneurysm is defined as an outward diastolic and systolic deformity of a thinned and scarred infarcted area (see Figs. 2.5 and 2.6). Its incidence ranges from 8% to 22%, it occurs predominantly (>90%) at the apex and after anterior MI, and it generally occurs within the first week post-AMI (20,21).
2D TTE has a sensitivity of 80% to 90% for detecting an LV aneurysm with improvement to >90% with cavity enhancing contrast studies (3,20,21).
RT3D TTE has a comparable diagnostic accuracy to MRI and left ventriculography for detection and
characterization of LV aneurysms, but it is superior to 2D imaging with a sensitivity, specificity, positive and negative predictive values of 100%, 91%, 89% and 100% versus 81%, 95%, 93% and 88%, respectively (22,23) (see Figs. 2.5C and 2.6B).
TEE has lower sensitivity than TTE for detecting apical LV aneurysms due to common foreshortening of the LV apex.
Patients with an LV aneurysm have an increased risk for thrombus formation and arterial embolism, which contributes to their increased risk for in-hospital and 1-year mortality.
Cardiomyopathy
Cardiomyopathy is defined as abnormally decreased LVEF (usually <40%) associated with global or segmental wall motion abnormalities and dilated LV.
Most common causes include coronary artery disease, hypertension, tachycardia mediated, alcohol abuse, and myocardial inflammatory or infiltrative processes.
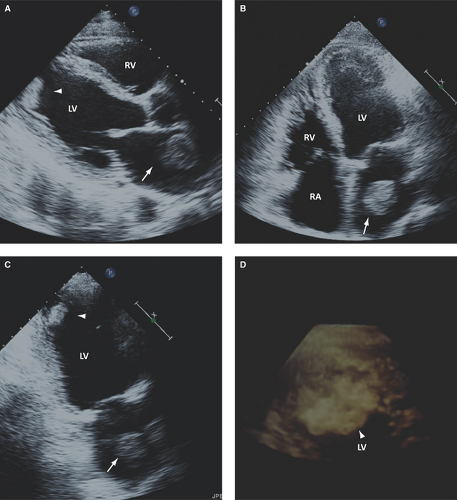
In patients with nonischemic dilated cardiomyopathy, a not well-determined increased prevalence of intracardiac thrombus and a 4% rate per year of arterial
embolism while in normal sinus rhythm have been reported (Fig. 16.2).
Endocarditis
Infective endocarditis of native or prosthetic valves is associated with an 11% to 44% rate of arterial embolism, with higher range rates among patients with vegetations >10 mm, Staphylococcus aureus endocarditis, or involvement of the mitral valve (24,25,26,27) (Fig. 16.7, see Figs. 13.1 to 13.4).
Noninfective endocarditis or Libman-Sacks endocarditis with vegetations is seen in at least 30% of patients with systemic lupus erythematosus (SLE) and in those with primary antiphospholipid antibody syndrome and is associated with a clinical and subclinical rate of arterial embolism of ≥30% (28,29) (see Figs. 13.6).
Prosthetic Heart Valves
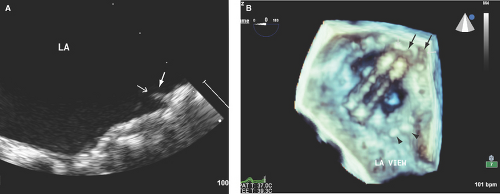
The true prevalence and incidence of prosthetic valves thrombosis is undefined. However, thrombosis with consequent thromboembolism does occur in patients with prosthetic heart valves predominantly in association with subtherapeutic anticoagulation or atrial fibrillation, more commonly with mechanical valves, and twice more common (≥50% of cases) for mitral valve prosthesis (30,31) (Fig. 16.3; see Fig. 11.11B).
The rate of thromboembolism in patients with mechanical heart valves is 4.4 per 100 patient-years without antithrombotic therapy, 2.2 per 100 patient-years with antiplatelet drugs, and 1 per 100 patient-years with warfarin therapy (30).
Fibrin strands, defined as thin, short, and mobile structures on the atrial side of prosthetic mitral valves (more commonly mechanical) and on the ventricular side of prosthetic aortic valves, can be detected in nearly 20% of patients (30,32). These structures are commonly incidentally detected, of uncertain pathogenesis, and difficult to differentiate from sewing or annuloplasty ring sutures. Their thromboembolic risk has not been fully determined.
Rheumatic Mitral Valve Stenosis
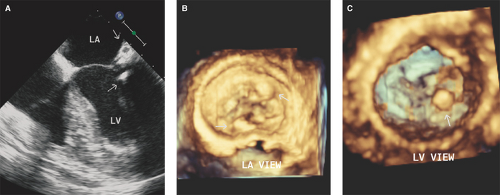
In patients with severe mitral stenosis, LA spontaneous echo contrast is detected by TEE in approximately 25% of patients in normal sinus rhythm and in >60% of patients in atrial fibrillation (33) (see Figs. 7.6 and 7.8A).
LA thrombi (predominantly formed in the LA appendage) occur in 7% to 15% of patients with mitral stenosis and are predominantly detected and characterized by 2D and RT3D TEE (34,35,36) (see Fig. 7.8).
LA thrombi can form in these patients while in normal sinus rhythm. An LA volume index (≥60 mL/m2) has been associated with thrombi in these subjects (37).
LA thrombi and pseudocontrast are both related to the severity of mitral stenosis, LA size, and atrial fibrillation (see Figs. 7.6 and 7.8B).
LA spontaneous echo contrast and thrombi can also occur in association with severe mitral annular calcification with or without inflow obstruction (Fig. 16.4).
During valve surgery, LA thrombi (more commonly formed in the appendage and LA posterior wall) are seen in 15% to 20% of patients with and without previous arterial embolism (35)
The prevalence of LA thrombi and pseudocontrast is lower in patients with moderate or severe MR (17%), as compared to those without significant MR (94%) (38).
Patients with symptomatic mitral stenosis have a rate of arterial embolism of 10% to 20% and pulmonary embolism of 10% (39).
Arterial embolism during mitral valvuloplasty occurs in up to 4% of patients and is most commonly due to LA thrombi (39,40). In those with a mitral valve morphology score >10, the rates of arterial embolism after
balloon valvuloplasty are higher (9% to 13%) (41). Associated aortic atheromatous disease or catheter-associated thrombi may contribute to this embolism.
Atrial or Ventricular Spontaneous Echocardiographic Contrast
LA or LV spontaneous echocardiographic contrast or “smoke” is caused by a low flow state leading to aggregation of blood cells.
Appears as swirling and smoke-like echoes and is more commonly seen in patients with atrial fibrillation or flutter, severe LV systolic dysfunction, LV aneurysm, rheumatic mitral valve stenosis, and low (≤20 cm/second) emptying atrial appendage velocities (Figs. 16.1A,B; see Figs. 2.1, 7.6, 7.8, and 11.11B).
It is uncommonly detected by TTE. In contrast, it is detected in 15% to 20% of patients undergoing TEE for all indications.
LA or LV spontaneous echo contrast is associated with an increased risk of LA or LV thrombi formation and future arterial embolism (Fig. 16.1C,D).
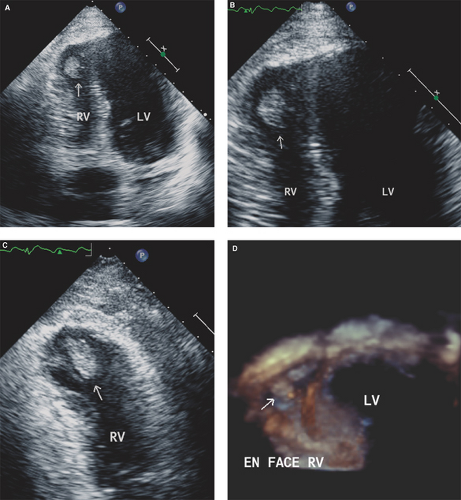
In patients with RV myocardial infarction or severe RV systolic dysfunction and in patients with atrial fibrillation, RV or RA spontaneous echo contrast and/or thrombus can occur and predispose patients to paradoxical embolism if an associated intracardiac shunt exists (Figs. 16.5 and 16.6).
Aortic Atheromatous Disease
Aortic atheromatous disease is an abnormal thickening of the intima and media of the aorta commonly associated with variable degrees of cholesterol crystals, fibrinous material, and/or thrombi deposition (Fig. 16.8). Its morphology is commonly categorized as follows:
Grade I: Minimal intimal thickening (<4 mm)
Grade II: Extensive or diffuse intimal thickening (≥4 mm)
Grade III: Sessile atheroma
Grade IV: Protruding atheroma
Grade V: Mobile and/or ulcerated atheroma
In retrospective series, the prevalence of aortic atheromatous disease by TEE in patients with stroke ranges from 14% to 33% as compared to 5% to 10% in case controls (42,43). In these series, aortic atheromas are predominantly seen in the descending thoracic aorta and lower rates in the aortic arch.
In controlled prospective series of patients with recent stroke undergoing TEE, aortic atheromatous disease has been detected in up to 70% of patients (nearly 40% of them with plaques ≥4 mm) (44).
Also, in these series, ulceration of aortic plaques occurs in 11% of patients with stroke as compared to 2% in those without stroke.