Intercostal, Intrapleural, and Peripheral Blockade of the Thorax and Abdomen
Dominic C. Harmon
George D. Shorten
Peripheral nerve blocks (PNBs) are applicable to virtually every thoracic and abdominal surgical procedure. Regional anesthesia and, in particular, PNBs can provide the basic foundation to allow a considerably less stressful operation, less deterioration in many physiologic functions, and improved postoperative analgesia.
For the purposes of this chapter, the thorax and abdomen will be considered separately. The thoracic component will concentrate on intercostal and paravertebral blocks, whereas the abdominal component will deal with ilioinguinal/iliohypogastric nerve block, rectus sheath block, and the recently described transversus abdominis plane (TAP) block. Intrapleural blockade applies to both regions. Ultrasound-guided techniques will be discussed where relevant.
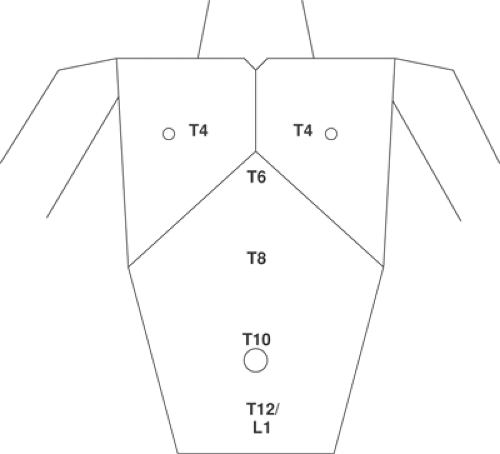
The trunk is divided into thorax and abdomen. The nerve supply to the thorax is derived from the anterior primary rami of T1 to T6. The nerve supply of the abdominal wall is derived from the anterior primary rami of T6 to L1. The sensory components of these nerves branch in the mid-axillary line. Important surface anatomic landmarks are (Fig. 16-1):
| T2 |
| T4 |
| T6 |
| T10 |
| T12 |
Analgesia In The Postoperative Period
Assessment of the impact of anesthetic and analgesic techniques commonly focuses on the incidence of mortality and major complications after major surgical procedures. Nevertheless, other postoperative adverse events such as pain, nausea and vomiting, and urinary retention may also impair patient comfort, recovery, and rehabilitation after minor and major surgical procedures. In addition, growing evidence suggests that acute postoperative events may lead to long-term consequences. For example, uncontrolled postoperative pain is related to the development of chronic pain syndromes (1); to postoperative myocardial ischaemia and infarction, which are risk factors for death from cardiac causes in the following months (2); and to postoperative cognitive decline, which can be persistent (3). As postoperative pain is often the predominant symptom, it can be considered an important outcome of surgery.
Pain is a key component in the alteration of lung function after thoracic and upper abdominal surgery. This highlights the importance of providing effective postoperative analgesia to reduce pulmonary complications and attenuate the stress response. Minor surgeries, such chest and abdominal wall procedures, are often performed on an outpatient basis. Postoperative analgesia in this setting is often not optimum (4), with associated adverse patient outcomes (4).
Peripheral nerve blockade has particular advantages over other analgesic techniques such as neuroaxial blocks and systemic opioids (5). This is also true in the setting of thoracic and abdominal surgery. Studies have examined the effects of intercostal blocks on lung volumes and gas flow rates following either abdominal or thoracic surgery. Most authors have used peak expiratory flow as a measure of the maximal expiratory effort that can be generated by a patient. When compared with opioid analgesia, intercostal block results in higher peak expiratory flows (6). This is true whether measured immediately after surgery or on the following day. In healthy volunteers, Jakobson found that bilateral intercostal nerve blocks with 0.25% bupivacaine or 0.5% etidocaine caused no change in the normal pattern of breathing (7). Minor changes were noted in several of the lung capacities and flows. Total lung capacity, forced vital capacity, and peak expiratory flow, all decreased by 4%. Functional residual capacity decreased by 8%, and peak expiratory airway pressure decreased by 7%.
Epidural analgesia is considered by many to be the best method of pain relief after major surgery. Although effective, side effects include hypotension, urinary retention, incomplete (or failed) block, and, in rare cases, paraplegia. Paravertebral block (PVB) is an alternative technique that may offer comparable analgesic effectiveness and a better side-effect profile. Davies and colleagues (8) performed a meta-analysis of all relevant randomized trials comparing PVB with epidural analgesia in thoracic surgery. Ten trials that had enrolled 520 thoracic surgery patients were identified. All of the trials were small (n<130), and none were blinded. There was no significant difference between PVB and epidural groups for pain scores. Pulmonary complications occurred less often with PVB (odds ratio [OR] 0.36; 0.14, 0.92). Urinary retention (OR 0.23; 0.10, 0.51), nausea and vomiting (OR 0.47; 0.24,0.53), and hypotension (OR 0.23; 0.11, 0.48), were less common with PVB. Rates of failed block were lower
in the PVB group, OR 0.28 (0.2, 0.6). The authors (8) concluded that PVB and epidural analgesia provide comparable pain relief after thoracic surgery, but PVB has a better side-effect profile and is associated with a reduction in pulmonary complications.
in the PVB group, OR 0.28 (0.2, 0.6). The authors (8) concluded that PVB and epidural analgesia provide comparable pain relief after thoracic surgery, but PVB has a better side-effect profile and is associated with a reduction in pulmonary complications.
Patient Comfort
Patient comfort during regional anesthesia relates to comfort during needle insertion, peripheral nerve stimulation if applicable, duration of surgery, and the postoperative period. Patient comfort during regional anesthesia is also an important teaching point for trainees (9). In ensuring patient comfort during regional anesthesia, several steps are involved. Some of these are outlined below.
Preoperative Preparation, Psychology and Communication
Psychology and communication play an important part in the success of any anesthetic technique (10). Patients’ previous experiences of regional anesthesia should be sought. Simple and precise language should be used.
Premedication
Pharmacologic premedication facilitates patient comfort during regional anesthesia performance (11). Advantages of premedication include improved patient satisfaction, acceptance, and cooperation. Disadvantages include unpredictable response, side effects, and interference with cooperation (12). In some cases, particularly with elderly patients and in ambulatory surgery, premedication is omitted.
When possible, premedication with nonopioid analgesics is optimum.
Sedation
Certain aspects of the regional technique often require both sedation and analgesia. Sedation alone in the presence of pain may cause confusion and restlessness. Conscious sedation is defined as a state of depressed consciousness that allows protective reflexes to be maintained, and the patient to respond appropriately to physical and verbal stimulation (13) (see also Chapter 8).
Regional Anesthetic Technique
In the performance of regional anesthesia, factors that impact on patient comfort should be optimized. The use of analgesics immediately prior to patient positioning can help increase patient comfort (14). Skilled, gentle digital palpation of surface anatomic landmarks is required. The pain on skin puncture is the most negative aspect of the patients’ experience of regional anesthesia (15). The smallest gauge needle (25–30 gauge) should be used for infiltration of skin and subcutaneous tissues.
Regional anesthesia can be a stressful patient experience, and all measures to establish and maintain patient comfort should be considered. The aim is to produce a relaxed patient who is comfortable and cooperative. In practice, achieving this ideal may be the most challenging part of regional anesthetic practice. Multiple techniques and drug regimes exist. No single algorithm or guideline can address the management challenges for a heterogenous patient population. A patient-centered approach, with individualized regimes, including procedures and drugs, will ensure a high standard of patient comfort without compromising safety.
Ultrasound-Guided Nerve Block Techniques
Ultrasound imaging is an attractive tool for regional anesthesia because of the many potential clinical benefits. Both cart-based and portable, compact ultrasound machines are now available and suited for nerve imaging. In theory, visual guidance can impart confidence to anesthesiologists, improve safety of patients, and enhance efficient time utilization in the operating room. Outcomes data to demonstrate convincingly that the clinical benefits of ultrasound are pending. There is no doubt that this imaging technology will be a valuable and enduring part of practice in regional anesthesia. In this chapter, where relevant, ultrasound-guided block techniques and available literature will be discussed.
General Principles of Ultrasound-guided Nerve Block Techniques
Certain general principles apply to the successful use of ultrasound to guide nerve block techniques:
The quality of ultrasonographic nerve images is dependent on the quality of the ultrasound machine and transducers, proper transducer selection (e.g., frequency) for each nerve location, the anesthesiologist’s familiarity and interpretation of sonographic anatomy pertinent to the block, and good eye-hand coordination to track needle movement during needle advancement.
Optimal patient positioning and sterile technique are required. This is particularly important for continuous catheter techniques. Sterile conducting gel and a sterile plastic sheath to fully cover the entire transducer should be used especially for catheter techniques.
Nerve localization by ultrasound can be combined with nerve stimulation. Ultrasonography provides anatomic information, while a motor response to nerve stimulation provides functional information about the nerve in question.
Observing local anesthetic spread is a valuable feature of ultrasound in addition to real-time visual guidance to navigate the needle toward the target nerve. Correct spread pattern predicts block success.
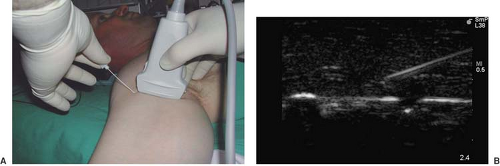 Figure 16-2. A: Long-axis needle approach with block needle parallel to the ultrasound beam. B: Ultrasound image of long-axis needle approach. The needle shaft and tip are visible. |
Two approaches are generally used to block peripheral nerves. The first approach aims to align and move the block needle parallel to the ultrasound beam (Fig. 16-2). The needle shaft and tip can thus be clearly visualized. This approach is preferred when it is important to track the needle tip at all times. The second approach aims to align and move the block needle perpendicular to the ultrasound beam (Fig. 16-3). In this case, the ultrasound image captures a transverse view of the needle, which is shown as a hyperechoic “dot” on the screen. Accurate moment-to-moment tracking of the needle tip location can be difficult, and needle tip position is often inferred indirectly by tissue movement and local anesthetic spread. This approach, however, is particularly useful for continuous catheter placement along the long axis of the nerve.
Block Preparation
The topic of equipment selection for neural blockade has been reviewed in detail, together with the general topic of perioperative management of patients undergoing neural blockade by Crews and Chan in Chapter 8. As for all regional anesthetic procedures, after checking that the emergency equipment is complete and in working order, intravenous (IV) access, electrocardiogram (ECG), pulse oximetry, and blood pressure monitoring are established. Asepsis is observed.
Intercostal Nerve Block
Anatomy
The intercostal nerves are the anterior primary rami of T1 through T11. T12 is not an intercostal nerve because it does not run a course between two ribs; it is more appropriately termed a subcostal nerve. Some of its fibers unite with fibers from the first lumbar nerve and are terminally represented as the iliohypogastric and ilioinguinal nerves.
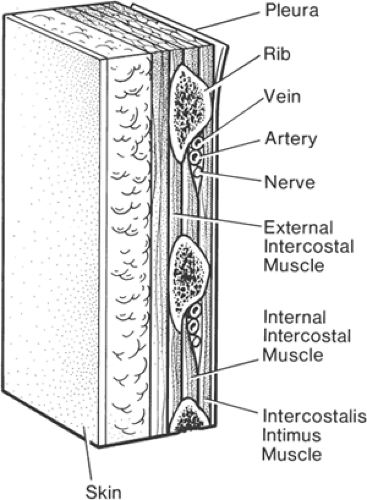
A typical intercostal nerve has four significant branches (Fig. 16-4). The first are the paired gray and white rami communicantes, which pass anteriorly to and from the sympathetic ganglion and chain. The second branch arises as the posterior cutaneous branch and supplies the skin and muscles in the paravertebral region. The third branch is the lateral cutaneous division, which arises just anterior to the midaxillary line. This branch is of most concern to the anesthesiologist because it immediately sends subcutaneous fibers coursing both posteriorly and anteriorly to supply skin of much of the chest and abdominal wall. The final branch of an intercostal nerve is the anterior cutaneous branch. In the upper five nerves, this branch terminates after penetrating the external intercostal and pectoralis major muscles to innervate the breast and front of the thorax. The lower six anterior cutaneous nerves terminate after piercing the sheath of the rectus abdominis muscle, to which they supply motor branches. Some final branches continue anteriorly and become superficial near the linea alba, to provide cutaneous innervation to the midline of the abdomen.
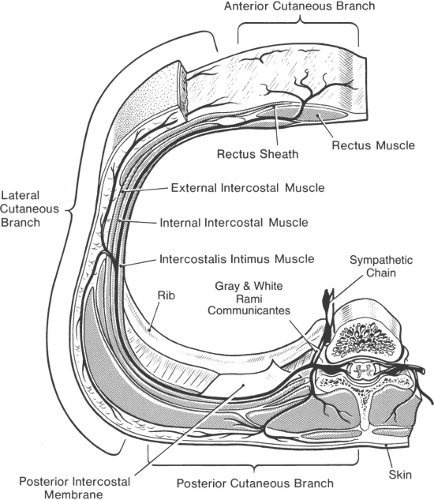 Figure 16-4. An intercostal nerve and its branches. Approximate area of skin supplied by branches is also shown. There is evidence, however, that local anesthetic injected near the lateral cutaneous branch diffuses posteriorly to reach the posterior cutaneous branch (see also Figure 16-9). Note also (a) the spinal nerves and dorsal root ganglia in the region of intervertebral foramen, with risk of perineurial spread into spinal fluid after intraneural injection in this region; (b) direct injection into an intervertebral foramen may reach spinal fluid by means of a dural cuff; (c) local anesthetic may gain access to epidural space by diffusing into an intervertebral foramen; (d) close to the midline, the intercostal nerve lies directly on the posterior intercostal membrane and pleura; and (e) paravertebrally, solution may diffuse to rami communicantes and sympathetic chain. |
Medial to the posterior angles of the ribs, the intercostal nerves lie between the pleura and the fascia of the internal intercostal muscle. This fascial layer is also known as the posterior intercostal membrane. In the paravertebral region, there is only fatty connective tissue between nerve and pleura. At the angle of the rib (6 to 8 cm from the spinous processes), the nerve comes to lie between the internal intercostal muscle and the intercostalis intimus muscle. At this position, the costal groove is broadest and deepest. Cadaver studies have shown that the nerve itself remains subcostal only 17% of the time, has most frequently (73%) moved inferiorly into the midzone between ribs, and is often branching at this point (16). The nerve is accompanied by intercostal veins and an artery, which lie superior to the nerve in the inferior groove of each rib (Fig. 16-5). The costal groove becomes a sharp inferior edge of the rib, about 5 to 8 cm anterolateral to the angle of the rib. At this point, the intercostal groove ceases to exist, the lateral cutaneous branch is given off (Fig. 16-4), and the intercostal nerve lies more inferiorly and moves toward the center of the intercostal space.
Technique
For technical ease of performance and for an optimal teaching or learning experience, the patient is best placed in a prone
position. The prone position is particularly favored if bilateral blocks are to be performed. A pillow is placed under the abdomen to decrease the lumbar lordosis and to accentuate the intercostal spaces posteriorly. The arms should be allowed to hang down from the edge of the block table to permit the scapula to rotate as far laterally as possible. If multiple levels are to be blocked then sedation is mandatory.
position. The prone position is particularly favored if bilateral blocks are to be performed. A pillow is placed under the abdomen to decrease the lumbar lordosis and to accentuate the intercostal spaces posteriorly. The arms should be allowed to hang down from the edge of the block table to permit the scapula to rotate as far laterally as possible. If multiple levels are to be blocked then sedation is mandatory.
The next step is the process of using skin markings (Fig. 16-6). First, a vertical line should be drawn along the posterior vertebral spines. The next step is to palpate laterally to the edge of the sacrospinalis group of muscles, where the ribs are most superficial. This distance is somewhat variable, depending on body size, muscle mass, and physique, but is usually 6 to 8 cm from the midline. Subsequent lines are drawn somewhat parallel to the first one, but with a trend to angle medially at the upper levels as the sacrospinalis muscles taper, so as to avoid the scapulae. The caudal end of the line should cross near the end of the shortened twelfth rib, which is generally easy to palpate. Then, by successively palpating and marking the inferior edge of each rib (or interspaces between ribs) a diagram is completed along these two vertical lines (Fig. 16-7). For abdominal surgery, six or seven (T5–T11 or T12) pairs of ribs are marked. For thoracic or other unilateral chest wall surgery, only the appropriate side and ribs are marked.
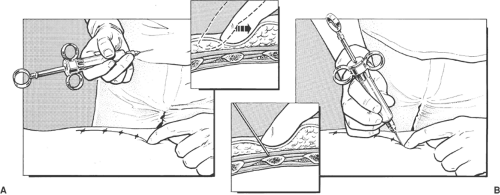
The anesthesiologist stands beside and behind the patient. The needle insertion point is infiltrated with local anesthetic using a 25-gauge needle. The needle insertion point is at the angle of the ribs. The ribs and intercostal spaces are thicker at the angle of the rib, allowing a larger margin of safety before pleura is contacted.
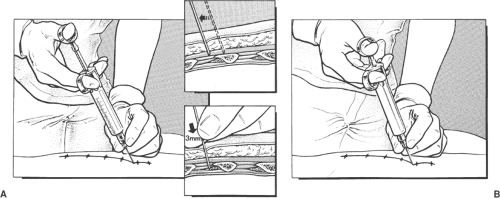
The index and third finger of left hand retract skin up and over rib. A 25-gauge 15-mm or a 23-gauge 25-mm needle is introduced in 20-degree cephalad orientation through the skin between the tip of the retracting fingers and advanced until it contacts rib. The left hand now holds needle hub and shaft between the thumb, index, and the middle fingers (Fig. 16-8). The left hand hypothenar eminence is firmly placed against the patient’s back. The needle and syringe move as a whole. This allows maximal control of the needle depth as the left hand walks the needle off the inferior margin of the rib and into the intercostal groove at a distance of 2 to 4 mm past the edge of the rib, where 3 to 5 mL of local anesthetic is injected after aspiration (Fig. 16-9). The average distance from posterior rib to pleura averages 8 mm (17), so advancing a small distance (2–3 mm) after walking off the rib is safe.
Intercostal nerve blocks can also be placed at the midaxillary line while the patient is lying supine. This position is considerably more convenient in many situations (e.g., after induction of general anesthesia), but there is less margin of safety, the costal groove no longer exists, the nerve has often split into several main branches, and the lateral cutaneous branch of the nerve could be missed by the injected solution. Computed tomographic studies show, however, that solutions spread readily along the subcostal groove for several centimeters and can come in contact with the origin or takeoff of this large branch (Figs. 16-4 and 16-9).
Continuous intercostal techniques have been described. Percutaneous techniques are associated with a failure rate of 10% to 30% (18,19). Catheters can also be placed under direct vision at the time of thoracotomy (20). A systematic review of randomized trials indicates that intercostal infusion provides analgesia that is at least as effective as an epidural and significantly better than systemic opioids alone (21).
Sonoanatomy of the Intercostal Block
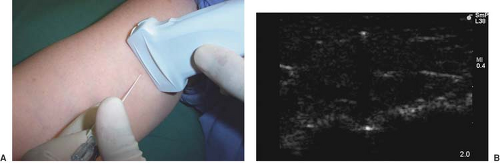
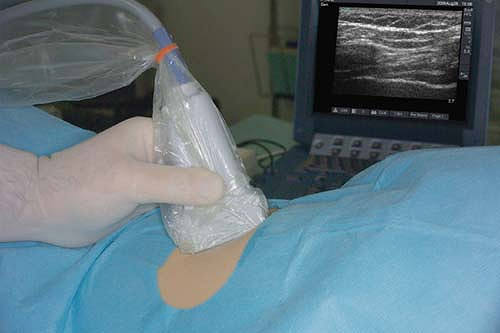
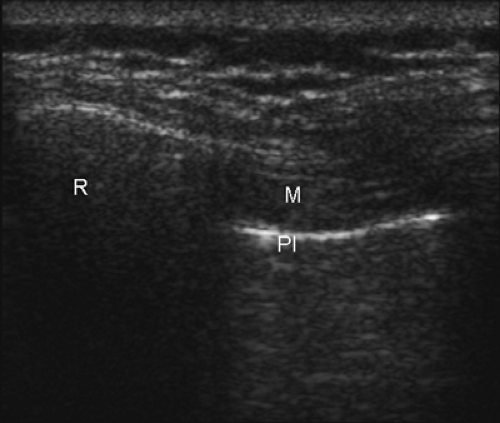
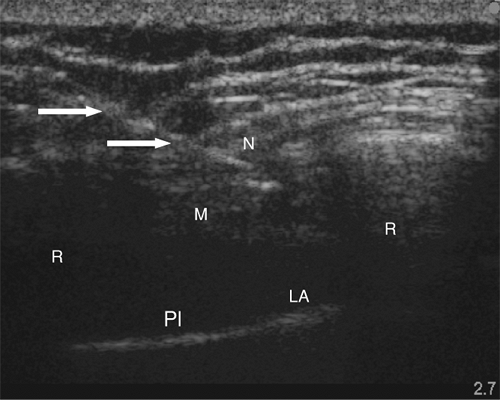
Only one report of ultrasound-guided intercostal nerve block appears in the literature (22). The authors report poor intercostal nerve visibility due to its proximity to the bony channel within which the nerve lies. The chest wall is best imaged in a coronal (vertical) plane (Fig. 16-10). Using a high-frequency transducer, the intercostal space can be visualized (Fig. 16-11). The ribs appear as dense, dark oval structures with a bright surface (periosteum). A dark shadow is cast deep to the rib on ultrasound, illustrating the phenomenon of echo shadowing. The pleura and lungs may also be visualized deep to the intercostal space between the echo shadows.
Ultrasound-guided Intercostal Block
The intercostal space is generally found at a depth of 2 to 3 cm from the skin. The uppermost rib is kept in the center of the field of view. The needle entry site is at the caudad edge of the linear transducer (Fig. 16-12). A 23-gauge needle is advanced under real-time ultrasound guidance, and local anesthetic is deposited along the needle entry path. A free-hand technique rather than the use of a needle guide is preferred. A 21-gauge needle is inserted parallel to the axis of the beam of the ultrasound transducer. The needle is attached to sterile extension tubing, which is connected to a 20-mL syringe and flushed with local anesthetic solution to remove all air from the system. It is then introduced at the caudad edge of the transducer and visualized along its entire path to the intercostal space. It is important not to advance the needle without proper visualization, achieving which may require needle or transducer adjustment.
The needle is advanced toward the inferior border of the rib. On contacting the rib, the needle is redirected inferiorly to pass no more than 0.5 cm beyond the inferior rib margin. Following a negative test aspiration, 2 to 5 mL of local anesthetic solution is injected.
Surgical Indications
Surgical intercostal block indications include upper abdominal and thoracic superficial procedures, such as insertion of
thoracotomy and gastrostomy tubes. Minor breast surgery (23,24), extracorporeal lithotripsy (25), and cardiac pacemaker insertion (26) have been described using intercostal blockade. Relatively few surgical procedures can be performed under intercostal nerve block alone; for major abdominal surgery, celiac plexus or splanchnic nerve block is required (see Chapters 42 and 45).
thoracotomy and gastrostomy tubes. Minor breast surgery (23,24), extracorporeal lithotripsy (25), and cardiac pacemaker insertion (26) have been described using intercostal blockade. Relatively few surgical procedures can be performed under intercostal nerve block alone; for major abdominal surgery, celiac plexus or splanchnic nerve block is required (see Chapters 42 and 45).
Nonsurgical Applications
Intercostal nerve block is extremely effective in providing pain relief for fractured ribs. Because vigorous palpation of broken ribs can be quite painful in the obese or in the patient with excessive local swelling, localization of the ribs and performance
of the block can be aided by ultrasound (22). Pleuritic pain and pain from flail chest also can be relieved in this manner. Herpes zoster pain may be relieved and even treated in this way. Intercostal nerve block can be helpful in the differential diagnosis of visceral versus abdominal wall pain. Chronic pain conditions such as intercostal neuralgia and tumor-related pain may also be treated.
of the block can be aided by ultrasound (22). Pleuritic pain and pain from flail chest also can be relieved in this manner. Herpes zoster pain may be relieved and even treated in this way. Intercostal nerve block can be helpful in the differential diagnosis of visceral versus abdominal wall pain. Chronic pain conditions such as intercostal neuralgia and tumor-related pain may also be treated.
Complications
Preoperative and postoperative chest films in 200 consecutive patients resulted in a pneumothorax incidence of 0.42% (1/2,610 intercostal nerve blocks) (27). Treatment of pneumothorax, by needle aspiration or merely by careful observation, is usually all that is needed.
A second complication relates to the toxic effects of absorbed local anesthetic and epinephrine following intercostal block. Blood levels of the anesthetic drug after intercostal and interpleural blockade are higher than after any other regional anesthetic procedure. Systemic toxic reactions rarely occur in patients having diagnostic or therapeutic blocks, because smaller volumes of more dilute solution of drug are used. Greater amounts of more concentrated drug are injected to provide complete motor and sensory block in the surgical patient.
Performance of intraoperative intrathoracic intercostal injection appears to be associated with a higher incidence of complications. Multiple cases of total spinal anesthesia have been reported after intrathoracic intercostal blockade conducted during surgery under general anesthesia (28,29). In most of these instances, the blocks were performed under direct vision at a site more medial than would likely be chosen for the percutaneous approach. Injection of local anesthetic into a dural root cuff or directly into nerve tissue itself, with extensive intrafascicular followed by spinal spread, has been proposed as the mechanism for the resultant widespread blockade, which manifests as hypotension and bradycardia, dilated pupils, and prolonged anesthesia and paralysis.
Interpleural Blockade
Anatomy
Interpleural block can provide analgesia over the chest wall and upper abdomen. Anesthesia is attained by diffusion of local anesthetic solution to nerves that run in proximity to the pleural surfaces. Anteriorly, laterally, and posteriorly, the parietal pleura is in close approximation to the intercostal nerves. Superiorly, the inferior roots of the brachial plexus pass a short distance over the cupola before reaching the first rib. Medially,
the sympathetic chain, splanchnic, phrenic, and vagus nerves are also adjacent. The epidural and subarachnoid spaces are at a greater distance and are generally not felt to be a site of local anesthetic action during interpleural anesthesia.
the sympathetic chain, splanchnic, phrenic, and vagus nerves are also adjacent. The epidural and subarachnoid spaces are at a greater distance and are generally not felt to be a site of local anesthetic action during interpleural anesthesia.
Technique
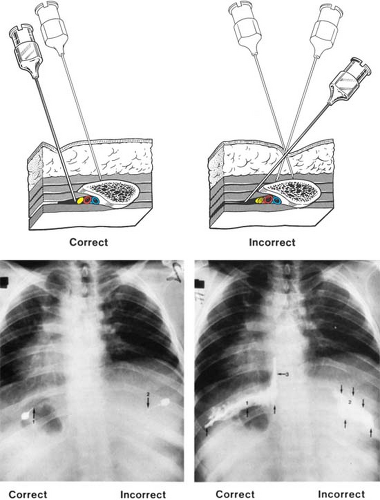
Spread of local anesthetic solution within the interpleural space is governed by gravity, the amount of volume injected, and the location of the catheter itself (30). The hallmark of this technique is detection of the negative interpleural pressure, so that placement should be performed either pre- or postoperatively in the awake patient, or during general anesthesia with the patient breathing spontaneously. Placement should be avoided during positive pressure ventilation, as the interpleural pressure is no longer negative, and the risk of pneumothorax and its evolution to tension pneumothorax is greatly increased (31).
The patient is placed in either the sitting, lateral, or prone position. After sterile prepping and draping, the skin is anesthetized at a point 8 to 10 cm lateral from the midline, overlying the top edge of a rib. Infiltration is carried deeper until the rib is contacted (Fig. 16-13). After recontacting the rib with a 16- or 18-gauge Tuohy needle, the needle is gently walked cephalad until it is felt to slide off the superior edge of the rib. A loss-of-resistance technique to positive pressure is used (32) (Fig. 16-13). Once in the interpleural space, the syringe is removed, and the interpleural catheter is gently passed 5 to 6 cm. The needle is removed, then an occlusive dressing is applied. If this technique is employed preoperatively, nitrous oxide should not be used during subsequent general anesthesia, because of the risk of significant expansion of the small pneumothorax that inevitably arises during even a brief interval of communication between the interpleural space and the atmosphere.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree