Links: Etiology | S/s | Tx | Forearm Ischemic Test |
An asymptomatic to potentially life-threatening syndrome of skeletal muscle breakdown and necrosis with release of intracellular contents into circulation including electrolytes and myoglobin. Due to acute necrosis of striated muscles, which release myoglobin (an O2 carrier with one hem moiety) and other cell constituents are released into circulation, leading to myoglobinuria (reddish-brown or smoky urine). Myoglobinuria is usually recognized by the pink or reddish hue of spun serum or by a positive dipstick test for Hb in the urine, with few or no RBC’s seen on microscopic examination. Myoglobin is filtered by the glomeruli and it may cause obstruction and renal dysfunction as it is toxic to renal cells.
Predisposing factors: dehydration, decr K & P, malnutrition, psychiatric dz, agitation, confusion, delirium. Endocrinopathy, shock, hypotension, hypoxia, acidosis, many medications.
Etiology: decr O2 delivery to muscles –> arterial occlusion, severe vasoconstriction, compression, hypokalemia, compartment syndrome.
Direct injury to muscle –> trauma, burns, toxins (alcoholic myopathy, heroin, ethylene glycol, snake bite, hornet sting, brown recluse spider bite, mercuric toluene), infections (viral, bacterial), primary muscle dz (dermatomyositis, muscular dystrophy), long-term confinement in the same position. Muscle overexertion (marathon runner).
Drugs (Ampho, antimalarials, amphetamine, statin, PCP, INH, heroin, ecstasy, diuretic, fibrates, colchicine, corticosteroid, AZT, others). Alcohol abuse or illicit drug use.
Incr Energy consumption –> sz, delirium tremens, electrocution, tetanus, malignant hyperthermia, heat stroke, drugs (sympathomimetics, PCP, succinylcholine, malignant neuroleptic syndrome), strenuous exercise.
Decr Energy storage or utilization –> decr P/ Ca/ K/ Na, carbo metabolism d/o (DKA, McArdle), lipid metabolism d/o (carnitine def, HMG CoA inhibitor meds), myxedema, hypothermia, AZT.
Genetic lipid, carb or purine metabolism d/o.
Child: The causes of rhabdomyolysis in children [viral myositis (38.2%) and trauma (25.7%)] are different from those in adult pt’s and the risk of acute renal failure seems to be much lower (Pediatrics 2006;118:2119-2125)….the most common sx’s included muscle pain (45%), fever (40%), sx’s of viral infection (39%), and muscle weakness (38%).
Forearm ischemic test: This test may be useful in assessing all pt’s with exercise intolerance. Demonstration of an absent lactate response and an exaggerated ammonia response to exercise points to glycogen storage disease (GSD) or myoadenylate deaminase deficiency (AMPD). This test is considered in patients presenting with postexertional myalgia, particularly when McArdle’s disease (myophosphorylase deficiency) or deaminase deficiency is suspected. The baseline serum lactate and ammonia are measured. By placing a blood pressure cuff and raising the pressure above systolic level, ischemia is induced. The pt then exercises by continuously squeezing a handgrip ergometer for approximately 1–2 minutes. The blood pressure cuff is then released, and blood from the antecubital vein is dawn. The serum lactate and ammonia are measured 0, 3, 5, 10, and 15 minutes post-exercise. No rise in lactate or ammonia when compared with baseline and a control subject is considered abnormal. This test now can be done without ischemia, but with longer exercise (e.g., 5–10 min), which makes it more tolerated. Best performed at a tertiary care center. In myopathies with intermittent sx’s, and especially those associated with myoglobinuria, there may be a defect in glycolysis. Best performed in a tertiary care center that has experience with the test.
Step #1: The pt fasts overnight. Get baseline ammonia & lactate from arm at rest w/o tourniquet. Place and stabilization a needle in a superficial antecubital vein of the arm to be exercised. A single blood sample of CK and sequential samples of lactate, pyruvate, and ammonia are obtained at intervals of 1, 2, 3, 5, and 10 minutes after removal of the blood pressure cuff.
Step #2: inflate cuff to 200 mmHg (or 20-30 above SBP, some say inflate to an intermediate between the systolic and DBP), then have pt do handgrip exercise with a ball (one hand grip/ second at least 75% of the maximum voluntary hand grip) to fatigue the forearm, most only last ~90sec. If an acute cramp develops, the cuff should be immediately deflated. Some have the forearm muscles are exercised by asking the pt to vigorously squeeze a sphygmomanometer bulb for 1 min.
Step #3: deflate cuff then draw labs again, then repeat draw in 2-5min. In variations of the test, blood is obtained at intervals of 1, 2, 4, 6, and 10 min for comparison with the baseline sample.
Interpretation: A normal response is a 3-fold rise in both lactate and ammonia. The simultaneous measurement of ammonia serves as a control, since it should also rise with exercise (pt’s with selective failure to increase ammonia may have myoadenylate deaminase deficiency). In pt’s with myophosphorylase deficiency or other glycolytic defects, the lactic acid rise will be absent or below normal, while the rise in ammonia will reach control values. If there is lack of effort, neither lactic acid nor ammonia will rise. In individuals with GSD (McArdles or other carb metab d/o), then ammonia levels rise, but lactate remains at baseline. In myoadenylate deaminase def, the lactate rises, but ammonia does not. Any abnormal result should be confirmed by enzyme analysis. If both normal, consider lipid metab d/o. In normal individuals after a good effort, a three- to fivefold rise in lactate is noted within the first one to three minutes. The rise in serum ammonia is similar, but somewhat slower and more gradual; ammonia reaches a peak at three to four minutes. The rise in lactate is <2-fold among pt’s with inborn errors of glycolysis/glycogenolysis; however, the increase in ammonia is normal in pt’s who have made sufficient effort during the test. Lactate production may be absent or diminished in phosphorylase, phosphofructokinase, debrancher, phosphoglycerate mutase, phosphoglycerate kinase, and LDH enzyme deficiencies. In the last condition, there is no rise in lactate levels, but pyruvate levels rise normally. The lactate curve is normal in acid maltase and in most cases of phosphorylase b kinase deficiencies. In pt’s with mitochondrial myopathies, there may be excessive production of lactate at submaximal levels of effort. With myoadenylate deaminase deficiency, there is absence of ammonia production with normal responses of venous lactate and pyruvate. The level of CK may rise in both glycogenolytic/glycolytic and fatty acid oxidation defects. The forearm ischemic exercise test is normal in defects of fatty acid metabolism as far as the lactate and ammonia curves are concerned.
Nuclear magnetic resonance (NMR) spectroscopy: a noninvasive method for the study of muscle metabolism, limited use since it is unavailable in most institutions. If used, the absence of intracellular acidification during exercise suggests a glycolytic defect. In mitochondrial defects, the ratio of inorganic phosphate to phosphocreatine at rest is elevated, with delayed resynthesis of phosphocreatine after exercise.
S/s Rhabdo: Classic triad of muscle pain + weakness + dark (pink, reddish-brown or black) urine (myoglobinuria). Also see swelling of muscles, cramps and bruising. Systemic sx’s include fever, malaise, nausea, emesis, confusion, agitation, delirium, anuria, chest pain, lower back pain, general malaise.
Ck ECG: R/o AMI and to monitor for cardiac arrhythmias due to electrolyte imbalances….findings suggesting severe manifestations include QRS interval widening and small P waves. Arrhythmias mostly caused by high levels of potassium.
Dx: by clinical presentation and typical laboratory findings.
Lab: Get muscle enzyme elevations (creatinine kinase [CK = CPK, see below], aldolase and lactic dehydrogenase). A CK (CK-MM) >5x the upper limit of normal or >1,000 U/L is usually used for dx, but commonly CK is elevated into the 10’s of thousands and often >100,000 IU/ml (vs normal “exertional” soreness gives CK of 3-5K). CK is more reliable than myoglobin for assessing the presence and intensity of muscle damage.
• Also have hemoconcentration, serum is clear (no hemoglobinemia), Cr is rising faster then BUN, early incr K, met acidosis with incr AG, incr P, decr Ca, incr uric acid. See marked elevations of potassium.
• See an initial hypocalcemia as calcium deposits in the injured muscle cells; hypercalcemia occasionally during the recovery phase from acute tubular necrosis. Often get a reversible hepatic dysfunction, but elevations in ALT, AST, and LDH may be due to muscle injury alone. Disseminated intravascular coagulation (DIC) can occur with increase in coagulation times (PTT), fibrin degradation products, and D-dimer with a decrease in platelets and fibrinogen.
U/A –> “cola-colored” reddish-brown, pH<5.5, +Protein, pigmented granular casts, renal epithelial cells, uric acid crystals, few to no RBC’s. Myoglobinuria does not occur w/o rhabdo, but rhabdo does not necessarily result in visible myoglobinuria as myoglogin is rapidly filtered from the blood stream. There are no erythrocytes on micro (hematuria), no red discoloration to plasma (hemolysis)….this is suggestive of injury from either hemoglobin or myoglobin. Extreme hyperuricemia may be present and may can cause acute uric acid nephropathy. Positive dipstick WITHOUT microscopic hematuria.
Creatine Kinase (CK, CPK): normal range: 0-130 U/L. CK is more specific and sensitive than AST and LD and more discriminating than aldolase (ALD) but AST is more significantly associated with inflammatory myopathy. Higher than expected variation of CK activity within the population suggests that the upper reference limit should be increased (Am Heart J 2007;154:655-661)….the 2.5th percentile was 40 IU/L and the 97.5th percentile was 460 IU/L (= 2.5 times higher than recommended by the assay manufacturer).
CK increased in: MI, myocarditis, rhabdo, myositis, crush injury/trauma, polymyositis, dermatomyositis, vigorous exercise (slight-to-significant increases in 14% to 100% of persons after extreme exercise; smaller increases in well-conditioned athletes), muscular dystrophy, myxedema, seizures, malignant hyperthermia syndrome, IM injections, CVA, PE / infarction, acute dissection of aorta.
Infections: Infections: Viral (HIV, EBV, influenza, picornaviruses, coxsackievirus, echovirus, adenoviruses). Bacterial (Staph, Strep, Clostridium, Borrelia). Fungal. Parasitic (trichinosis, toxoplasmosis, schistosomiasis, cysticercosis).
Drugs: alcohol, cocaine, halothane (malignant hyperthermia), ipecac. Statin drugs in certain pt’s.
CK decreased in: Steroids, decreased muscle mass, connective tissue disorders, alcoholic liver disease, metastatic neoplasms.
CK-BB Isoenzyme: Elevated in cerebrovascular accident, subarachnoid hemorrhage, neoplasms (prostate, GI tract, brain, ovary, breast, lung), severe shock, bowel infarction, hypothermia, meningitis.
CK-MB Isoenzyme: Elevated in MI, myocarditis, pericarditis, muscular dystrophy, cardiac defibrillation, cardiac surgery, extensive rhabdomyolysis, strenuous exercise (marathon runners), mixed connective tissue disease, cardiomyopathy, hypothermia. CK-MB exists in the blood in two subforms. MB2 is released from cardiac cells and converted in the blood to MB1. Rapid assay of CK-MB subforms can detect MI (CK-MB2 e1.0 U/L, with a ratio of CK-MB2/CK-MB1 e1.5) within 6 hours of onset of symptoms.
Macro CK: Generally causes only a minimal elevation (<500 IU/l) in CK or a high CKMB/CK ratio with no elevation of the total CK. Two types of macro CK have been described.
• Macro CK type 1 is an isoenzyme–Ig complex created via an antigen–antibody reaction. The most common form of type 1 macro CK is a CKBB–IgG complex. Less is known about the composition of macro CK type 2. It is believed to be of mitochondrial rather than cytoplasmic origin. Macro CK is a rare but important cause for a raised serum creatine kinase concentration (Rheumatology 2003:42:186-188). Diagnosis can most readily be made by CK electrophoresis, immunoinhibition and chromatographic techniques. When CK electrophoresis is used, the diagnosis is confirmed through the different electrophoretic mobility of the macro CK compared with the other CK isoenzymes.
Studies published to date have reported the prevalence rate for macro CK type 1 to be 0.43–1.2%. Macro CK type 1 may occur in healthy individuals and in children and is most commonly found in women and patients over the age of 50yr. It is associated with hypothyroidism, neoplasia, autoimmune disease, myositis and cardiovascular disease.
• Macro CK type 2 has a reported prevalence rate of 0.5–3.7%, the higher rates being reported from in-patient populations. Macro CK type 2 is less readily identified and is therefore likely to be under-reported as the total CK level will be raised in only 10–20% of cases. In the remaining 80–90% the main potential marker for the presence of macro CK type 2 is an elevation in the CKMB isoenzyme above 50% of the total CK concentration; even in individuals who have had a recent myocardial infarction this isoenzyme rarely makes up more than 30% of the total CK concentration. Unlike type 1, macro CK type 2 has been reported in predominantly ill patients, most commonly in association with malignancy (colonic carcinoma) and liver disease.
Tx of Rhabdo: The most effective tx is to rapidly restore intravascular volume with NS (0.9%) at 1-1.5 Liters/hr with a goal of urine output of 200-300ml/hr until CPK <1000 U/ml and myoglobinuria stops (clear urine or urine dipstick negative for blood). Once stable change to 3% saline with 5% glucose at 200 to 400 ml/hr. May need to give up to 10L/d of fluids.
• If oliguria use D5W with 100ml bicarb (1-3 amps bicarb /L).
• Can also use ½NS (77mmol/L Na) + ½ amp bicarb (75 mmol Na to alkalinize the urine and correct the acidosis). Goal is to alkalinize the urine to a pH >6.5 to decr toxicity of myoglobin and promote osmotic diuresis. If the urine output is >20ml/hr: also give 15% Mannitol @10ml/hr or use 100ml of 25% solution IV over 15min PRN or add 10g/L to the IV bag with bicarb. If the pt continues to be oliguric (UO <400 ml/day), the infusion should be discontinued and the pt treated conservatively for ARF.
• Check serum potassium level every 4 hours if creatine kinase (CK) level > 60,000 units/L or suspected toxicity cardiac monitoring if serum potassium level > 6 mEq/L (6 mmol/L) or rapidly rising, or if ECG abnormalities
• If incr K give Kayexalate 30mg PO. Potassium levels can also reduced with IV insulin plus glucose and other strategies. Avoid lactate or potassium containing fluids. Loop diuretics may be beneficial to increase tubular flow and to excrete excess calcium. Allopurinol may be beneficial to decrease the production of uric acid and act as a free radical scavenger. May need dialysis if ARF (oliguric ATN), severe incr K or acidosis. Consider a single dose of Diamox.
• Hypocalcemia should only be corrected if symptomatic (tetany, seizures) or presence of severe hyperkalemia. Avoid hypercalcemia….avoid repleting Ca initially unless sx’s as tends to rebound.
• Dialysis if severe renal failure or inability to correct volume overload, acidosis, or hyperkalemia.
• Fasciotomy if increased compartment pressure (risk for muscle necrosis or nerve damage).
• Elderly patients should be admitted to ICU for close monitoring of vital signs, intake and output and detection of fluid overload.
Mannitol works in four ways: osmotic agent that pulls fluid from interstitial compartment to decreases swelling, increases renal blood flow, osmotic diuretic to increase UO (prevent myoglobin casts), scavenges free radical.
Prognosis: course of disease varies depending on underlying cause. Full recovery expected with early diagnosis and treatment of complications. Pattern of creatine kinase (CK) level elevation: rise within 12 hours of muscle injury, peaks in 1-3 days, declines 3-5 days after cessation of muscle injury, half-life of CK is 1.5 days. Risk of renal failure increases with CK > 5,000 units/L in trauma patients (19% vs 8% in patients with CK < 5,000 units/L) ( J Trauma 2004;56(6):1191).
ICD-9 code:
728.88 rhabdomyolysis
ICD-10 codes:
M62.8 other specified disorders of muscle optional subclassification codes to indicate site of involvement
0 multiple sites 1 shoulder region 2 upper arm
3 forearm 4 hand
5 pelvic region and thigh 6 lower leg
7 ankle and foot 8 other 9 site unspecified
T79.6 traumatic ischemia of muscle
***Ref: (Myoglobinuria. Nephrol Clin. 2000;18:215-43) (Rhabdomyolysis. Crit Care Clin. 1999;15:415-28) (Acute exertional rhabdomyolysis. Am Fam Physician. 1995;52:502-6) (Rhabdomyolysis. J of Am Soc of Nephrol 2000;11:8) (Nontraumatic rhabdomyolysis. Acute Renal Failure: A Companion to Brenner & Rector’s The Kidney. Molitoris BA, Finn WF, Eds. W.B. Saunders, Philadelphia, 2001, p 220)
Adrenal Insufficiency (AI):
Links: Info, Etiology | S/s | Primary (Addisons) | Secondary | W/u | Tx of Crisis and Chronic Pt’s |
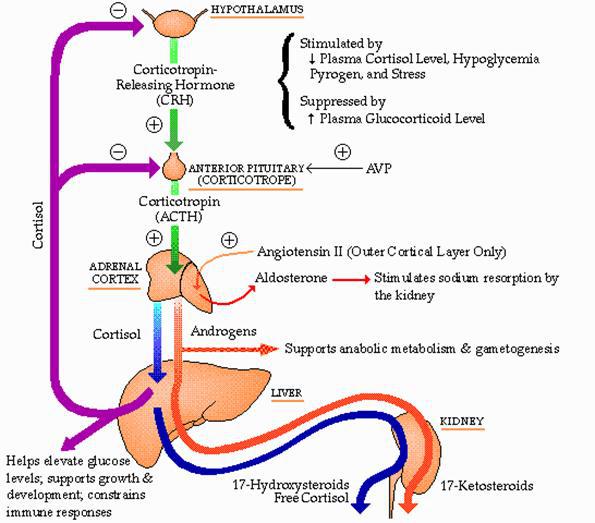
About 12 people in 100,000 have primary adrenal insufficiency. Congenital adrenal hyperplasia may affect 1 in 16,000 people. The initial task in the Ddx of glucocorticoid deficiency is to define whether the process is ACTH dependent. This distinction is best made on the basis of plasma ACTH concentrations measured at the time of glucocorticoid deficiency (before tx with glucocorticoid has been initiated). ACTH concentrations in or below the normal range imply an ACTH-dependent process. ACTH concentrations above the normal range imply an ACTH-independent process. The integrity of the hypothalamic-pituitary-adrenal axis can be established by measuring a morning cortisol (nl if >18 mcg/dL), except in high estrogen states (pregnancy estrogen use) in which cortisol-binding globulin and therefor total cortisol levels are elevated or with a peak value of >18 mcg/dL with cosyntropin stimulation test.
Primary (loss of adrenal glands) Adrenal Insufficiency: Autoimmune destruction (Addison disease). Surgery or trauma. Infectious destruction (tuberculosis, fungi). Hemorrhagic destruction (e.g., Waterhouse-Friderichsen syndrome of meningococcemia, purpura, and acute adrenal hemorrhage).
 Secondary Adrenal Insufficiency: Pituitary insufficiency. Inadequate ACTH production. Withdrawal of exogenous glucocorticoids (common, iatrogenic).
Secondary Adrenal Insufficiency: Pituitary insufficiency. Inadequate ACTH production. Withdrawal of exogenous glucocorticoids (common, iatrogenic).
ACTH-dependent glucocorticoid deficiency: implies disordered function of the hypothalamus and/or pituitary gland leading to ACTH deficiency.
Causes include hypothalamic-pituitary-adrenal suppression due to exogenous (glucocorticoid or ACTH) or endogenous –cure of Cushing’s syndrome, hypothalamic-pituitary lesions (primary pituitary tumor or metastatic tumor), craniopharyngioma, infection (TB, actinomycosis, nocardiosis), sarcoid, head trauma or isolated ACTH deficiency.
ACTH-independent glucocorticoid deficiency: caused by disordered adrenal function, such as destruction of the gland by an infectious process like tuberculosis. This distinction is best made on the basis of plasma ACTH concentrations measured at the time of glucocorticoid deficiency (i.e., before tx with glucocorticoid has been initiated). ACTH concentrations in or below the normal range imply an ACTH-dependent process. ACTH concentrations above the normal range imply an ACTH-independent process.
Causes include: TB. Autoimmune (idiopathic) and other rare causes such as fungal infection, adrenal hemorrhage, metastases, sarcoidosis, amyloidosis, adrenoleukodystrophy, adrenomyeloneuropathy, HIV infection, congenital adrenal hyperplasia.
Drug-induced AI: Drugs that may cause adrenal insufficiency by inhibiting cortisol biosynthesis, particularly in individuals with limited pituitary and/or adrenal reserve, include aminoglutethimide (antiepileptic), etomidate (anesthetic-sedative), ketoconazole (antimycotic) and metyrapone. Drugs that accelerate the metabolism of cortisol and most synthetic glucocorticoids by inducing hepatic mixed-function oxygenase enzymes, such as phenytoin, barbiturates, and rifampicin can also cause adrenal insufficiency in patients with limited pituitary or adrenal reserve, as well as those who are on replacement therapy with glucocorticoids. Some of novel tyrosine kinase-targeting drugs (e.g. sunitinib) have been shown in animal studies to cause adrenal dysfunction and hemorrhage. Megestrol (Megace), prescribed to stimulate appetite, has also been shown to cause adrenal insufficiency, as well as severe hyperglycemia (J Endocrinol Invest. 2006;29(2):136-40).
S/s: fatigue, weakness, mental depression, anorexia, wt loss, dizzy, N/V/D, muscle cramps, hypotension.
Primary AI:, decr Na, incr K, hypoglycemia, normocytic anemia, eosinophilia, lymphocytosis, salt craving (20%), Non-anion gap metabolic acidosis, hyperpigmentation, H-A. Calcified ear cartilage if long standing.
Skin: Addison’s can present with tanning of the skin that may be patchy or even all over the body….Characteristic sites of tanning are skin creases (e.g. of the hands) and the inside of the cheek (buccal mucosa). See Primary (Addisons) |
Acute AI: should be suspected when hypotension occurs in a pt with chronic adrenal insufficiency or in association with any of the known causes of adrenal insufficiency. If acute onset of hypotension, shock and volume repletion that is unexplained and catecholamine resistant always consider this and tx with steroids (dexamethasone rather than hydrocortisone otherwise can’t work-up until out of system for days), do not wait for lab confirmatory tests. See Tx |
Ddx: adrenal atrophy after glucocorticoid therapy.
Secondary AI: Hypoglycemia. Fatigue. Wt loss. No change in K or Na as RAS is intact. Decreased pigmentation (not making ACTH). See Secondary |
Primary Adrenal Insufficiency (AI) (Addison Disease):
Most commonly (85%) due to adrenal atrophy from autoimmune adrenalitis (destruction of cells in the adrenal cortex) in 80% (have +Ab to adrenal or 21-hydroxylase enzyme, which is involved in the synthesis of glucocorticoids and aldosterone). Can also be due to TB in 15%, Adrenal mets in 10% (lung, breast, kidney), systemic fungal infection (histo/ crypto/ blastomycosis), AIDS (CMV, protozoa, Kaposi’s), lymphoma (infiltrative disease), hemorrhage/ septicemia (meningococcemia or pseudomonas septicemia). Need ~90% of the gland is destroyed before sx’s. Have increased incidence of thyroiditis. In autoimmune adrenalitis, cytotoxic lymphocytes slowly destroy the adrenal cortex. From 50-70% of pt’s also have another autoimmune dz such as DM or hypothyroid (polyendocrine deficiency syndrome).
Abrupt onset–> adrenal hemorrhage/ necrosis/ thrombosis (meningococcal and other sepsis, coagulation d/o, Warfarin tx, antiphospholipid Ab syndrome, trauma).
Waterhouse-Friderichsen syndrome: is characterized by “petechial rash, coagulopathy, cardiovascular collapse and bilateral adrenal hemorrhage that is primarily associated with fulminant meningococcemia. Three cases of community-acquired invasive Staphylococcus aureus infection in infants leading to Waterhouse-Friderichsen syndrome (NEJM 2005;353:1245-1251) (2 were MRSA, all 3 died).
S/s: Sx’s are often vague, the most frequent complaints are chronic weakness and fatigue, N/V, hyperpigmentation and (less  frequently) acute abdomen. Salt craving or excessive intake of licorice, to substitute for the deficiency in aldosterone production, is often present (it should be
frequently) acute abdomen. Salt craving or excessive intake of licorice, to substitute for the deficiency in aldosterone production, is often present (it should be  noted that licorice candy often does not contain actual licorice but only flavor; however, licorice root is widely available). Addison’s is often associated with other autoimmune d/o’s such as autoimmune thyroid disease, premature ovarian failure, type I diabetes mellitus, vitiligo, alopecia and coeliac disease (a cluster known as autoimmune polyendocrine syndrome type II).
noted that licorice candy often does not contain actual licorice but only flavor; however, licorice root is widely available). Addison’s is often associated with other autoimmune d/o’s such as autoimmune thyroid disease, premature ovarian failure, type I diabetes mellitus, vitiligo, alopecia and coeliac disease (a cluster known as autoimmune polyendocrine syndrome type II).
• A 58-year-old woman gets so anxious she watching her sports team (Manchester United) trying to hang on to a precarious lead in the dying minutes of a match that she has admitted for treatment of Addisonian crisis (BMJ 2011;online December 15)…..the first description of Manchester United-induced Addisonian crisis.
Manifestations of Primary Adrenal Insufficiency: Weakness, tiredness, fatigue 100%. Anorexia 100%. GI symptoms 92%. Nausea 86%. Vomiting 75%. Constipation 33%. Abdominal pain 31%. Diarrhea 16%. Salt craving 16%. Postural dizziness 12%. Muscle or joint pains 6–13%. Weight loss 100%. Hyperpigmentation 94%. Hypotension (<110 mm Hg systolic) 88–94%. Vitiligo 10–20%. Auricular calcification 5%. Autoimmune Addison’s disease in women, especially when it goes undiagnosed, raises the risk of preterm birth, low birth weight, and cesarean delivery (J Clin Endocrin Metab. Posted online September 22, 2010)…..”Careful monitoring of AAD women is needed to observe signs of fetal growth retardation with potentially harmful short- and long-term consequences for the infant”.
Laboratory Finding: Electrolyte disturbances 92%. Hyponatremia 88%. Hyperkalemia 64%. Hypercalcemia 6%. Azotemia 55%. Anemia 40%. Eosinophilia 17%. (The Most Common Inpatient Problems in Internal Medicine. 2007, Elsevier)
Lab: Incr serum ACTH/ Renin/ K/ HCT/ eosinophils/ BUN.
Decr: cortisol/ pH, Na/ Bicarb/ fasting BS/ WBC with relative lymphocytosis. Pt’s with Addison’s disease can be expected to have a normal lifespan. See W/u |
Tx: see below. Tx |
Secondary Adrenal Insufficiency (AI):
Due to deficiency in ACTH. Often has diabetes insipidus.
Has three causes: adrenal suppression after withdrawal of exogenous glucocorticoid (after 2 weeks use) or ACTH administration, adrenal suppression after the correction of endogenous glucocorticoid hypersecretion, and abnormalities of the hypothalamus or pituitary gland leading to ACTH deficiency. Secondary AI can become manifest shortly after the cessation of corticosteroid therapy or mo’s later in a stressful setting such as after an injury or a surgical procedure. Any lesion of the hypothalamus or pituitary gland can lead to secondary AI; examples include space-occupying lesions such as craniopharyngioma, pituitary adenoma, metastases from distant malignancies, sarcoidosis, and infections with fungi (Nocardia, Actinomyces) or the tubercle bacillus. Trauma to the stalk or its blood supply also can lead to adrenal insufficiency. A deficiency of ACTH in the absence of any of these underlying causes is rare. Inhaled corticosteroids appear to increase the risk of adrenal insufficiency, particularly those who are also on intermittent oral corticosteroid therapy (Thorax 2006;61:405-408)… Pt’s on oral corticosteroids had a 2.0-fold increased risk of adrenal insufficiency per course of tx per year, and the risk was also dose-related. When an inhaled corticosteroid was also prescribed, the risk increased to 3.4-fold.
Slow onset–> pituitary/ met neoplasia, craniopharyngioma, pituitary trauma/ radiation, lymphocytic hypophysitis, sarcoid, histiocytosis X, empty sella syndrome, hypothalamic tumor, long term glucocorticoid therapy.
Abrupt onset–> postpartum pituitary necrosis (Sheehan’s), necrosis of pituitary macroadenoma, head trauma causing lesion of pituitary, surgical trauma (transient).
PP: The problem is insufficient ACTH. Low POM-C, low melaocyst stimulating hormone (MSH) which means loss of pigmentation. All of the usual findings of low cortisol. Cortex is intact, and aldosterone is normal. Angiotensin II stimulates zona glomerulosa. Volume and potassium are normal.
S/s: pale skin (hypogigmented) w/o marked anemia, amenorrhea, decr libido, scant axillary/ pubic hair, small testicles, secondary hypothyroidism, H-A, visual sx’s, DI, prepubertal growth deficit, delayed puberty. See W/u |
In primary AI the skin/mucosa is darker, K is high, Na is low and associated with primary hypothyroidism, type 1 DM, vitiligo and adrenoleukodystrophy.
In secondary AI the skin in pale, K is normal, is normal or low and is associated with central hypogonadisms / hypothyroidism, GH def, diabetes insipidus, HA’s and visual abn’s.
Tx: During recovery, the patient requires coverage. Hydrocortisone in the morning. Extra pills during any illness.
W/u: of acute AI: (w/u of “stable”, chronic AI, see below).
When acute AI it is suspected: immediately measure the plasma cortisol level (non-stimulated)…..ranges between 20 and 120 µg/dL during severe stress or shock in pt’s with normal adrenal function, a plasma cortisol concentration of <20 µg/dL favors a dx of adrenal insufficiency. Often see a low serum Na level and elevation of BUN & potassium levels. In pt’s severely ill with serum albumin <2.5g/L the total cortisol is often <18 ug/dL, thus less reliable index for AI. If the dx is seriously entertained, the pt should be given 100mg of hydrocortisone IV, followed by 10 mg/hr IV until the cortisol result is obtained. Definitive eval requires plasma cortisol levels drawn before and 60 minutes after administration of 0.25mg of synthetic ACTH (Cortrosyn). The pt who is thought likely to have AI (e.g., someone already hyponatremic or hypotensive) should be tested somewhat differently. A morning cortisol measurement and ACTH measurement should be taken, and then, to avoid adrenal collapse, the pt can be given 0.5 mg of dexamethasone BID and 0.1 mg of fludrocortisone daily during the testing. Cortisol is measured at 30 and 60 minutes after administration of a 0.25 mg dose of synthetic ACTH. A plasma cortisol level above 18 g/dl establishes normal adrenal function; however, if the initial measurements showed cortisol to be low and it remains low and the ACTH to be above 100 pg/ml, the dx of primary AI is established.
Chronic AI W/u: Evaluation of adrenal function: The principal tests used to evaluate adrenal function include measurements of plasma ACTH and cortisol and of urinary 17-OHS and 24-hour urinary free cortisol (Diagnosis of Adrenal Insufficiency. Ann Internal Med 2003;139:194-204). MRI of the brain is usually performed to exclude the presence of a sellar or suprasellar mass. In women, the response to ACTH is affected by the use of oral contraceptives, and thus OCs should be discontinued prior to testing to avoid underestimating hypocortisolism in these patients (J Clin Endocrinol Metab. 2007;92(4):1326-1333).
Step #1: Baseline cortisol +ACTH.
Basal morning (8-9AM) plasma cortisol should reflect physiologic peak, if <5 mcg/dL (140 nmol/L) then presumptive evidence of adrenal insufficiency. If 5-10, probable (get CRH test), if >18-20 mcg/dL (550 nmol/L) then clearly rules out adrenal insufficiency and no further w/u needed. Normal resting values of plasma cortisol are between 10 and 25 mg/dl in the morning and between 2 and 10 mg/dl at night.
Notes: The plasma cortisol level is altered by changes in the amount of cortisol-binding globulin, which generally parallel changes in the level of thyroxine-binding globulin. Both proteins are raised by increased estrogen levels and lowered by hepatic disease. Resting levels of cortisol are often less significant than the responses of the hormone to physiologic stimuli. Thus, plasma cortisol levels are usually measured after administration of exogenous steroid to determine whether the pituitary-adrenal axis is susceptible to feedback inhibition (i.e., whether it is suppressible) and after ACTH administration to see whether the adrenal responds to stimulation.
Plasma ACTH: (8-9AM) If primary AI then high, >100-150 pg/ml (22 pmol/L). If secondary adrenal insufficiency ACTH can be either low or normal (<60 pg/mL) (when serum cortisol is reduced). If both cortisol and ACTH are low then = pituitary insufficiency.
Step #2: determine pituitary-adrenal suppression. If AM cortisol <1820 ¼g/dL and Al suspected, perform ACTH stimulation test.
1. To measure adrenal response: Cosyntropin (ACTH) Stimulation Test = Cosyntropin (ACTH) Stim Test | 0.25 mg of cosyntropin (synthetic ACTH) is given IV. The plasma cortisol is measured at 0 and 60 minutes; a value of 20 mcg/dl at any time during the test denotes normal adrenal responsiveness.
Low ACTH level (<10–20 pg/mL) = Secondary or tertiary Al.
High ACTH level (>100 pg/mL) = Primary Al.
2. To determine pituitary-adrenal suppression: Dexamethasone Suppression Test: 1 mg of dexamethasone (a cortisol analogue) is given orally at 11:00 P.M., and the plasma cortisol level is measured at 8:00 A.M. the next day. A value of < 5 g/dl is normal.
3. To assess pituitary-adrenal reserve: Short Metyrapone Test: Metyrapone is no longer available in the US in 2007. Metyrapone inhibits 11-beta-hydroxylase, resulting in blockage of the conversion of 11-deoxycortisol (compound S) to cortisol. It is used to measure the ability of the HPA axis to respond to an acute reduction in serum cortisol levels. As cortisol serum levels fall, normal persons show a release of ACTH, which results in a further increase in serum 11-deoxycortisol levels. The test is used to differentiate ACTH-secreting pituitary tumor from adrenal tumors or from paraneoplastic syndromes due to ectopic ACTH secreting tumors. It also can be used to distinguish normal persons from pt’s with secondary adrenal insufficiency, but it will not differentiate primary from secondary adrenal insufficiency. A normal hypothalamic-pituitary axis is needed for a normal response.
Protocol: Before testing the pt, a normal response to exogenous ACTH should be demonstrated, in order to reduce the risk of adrenal crisis in pt’s with primary adrenal insufficiency. The single dose test can be performed on outpt’s, but the pt should be monitored during testing. Usually the dose of metyrapone is given at midnight with specimen collection occurring the following morning. Metyrapone 30 mg/kg (max dose 3 g) is given orally at 11-12 PM at night. Since metyrapone can cause gastric irritation, mild or a light snack is given with the oral dose; this also helps delay metyrapone absorption. A baseline serum 11-deoxycortisol, cortisol and ACTH is collected prior to metyrapone administration. A repeat sample is collected at 8 AM the following morning.
Interpretation: normal persons show increased 11-deoxycortisol levels (> 7 mcg/dL, normally <= 1 mcg/dL), reduced cortisol (< 3 mcg/dL) and increased ACTH (> 150 pg/mL). Pt’s with hypopituitarism, Cushing’s disease due to adrenal tumors or nonendocrine tumors secreting ACTH will have no response or an impaired response to the metyrapone. Reduced response is seen in drug therapy with drugs that incr hepatic metyrapone metabolism (phenytoin, phenobarbital) or with steroid therapy (glucocorticoids, oral contraceptives, estrogens). An exaggerated response can be seen in pituitary ACTH secreting adenomas (pituitary Cushing’s dz).
ICU Pt’s: Free cortisol, not total cortisol, should be measured in critically ill hypoproteinemic pt’s, as >90% of circulating cortisol in human serum is protein-bound. A significant percentage of critically ill pt’s with hypoproteinemia may have below normal concentrations of serum total cortisol (and cosyntropin-stimulated serum total cortisol) in spite of having normal adrenal function. Measuring serum free cortisol concentrations may prevent unnecessary glucocorticoid therapy (NEJM 2004;350:1629-38).
Alternative method of measuring pituitary-adrenal reserve: involves the IV administration of insulin, 0.15 U/kg. The ensuing hypoglycemia should elicit both cortisol (normal, 20 mg/dl) and growth hormone (> 8 ng/ml) responses. This test should be carried out in the presence of a physician prepared to administer 25 ml of 50 percent glucose intravenously in case of a hypoglycemic reaction. To measure pituitary reserve of ACTH, ovine CRH can be administered in a dose of 1 mg/kg body weight. Both ACTH and plasma cortisol levels rise in normal individuals. This test can be performed at any time of day, but the sensitivity is greatest in the afternoon.
Urinary 17-OHS level: The urinary 17-OHS measurement has the advantage of integrating the secretion of cortisol during a 24-hour period, but its usefulness is limited because the level is elevated by obesity and depressed by hepatic and renal disease. Moreover, the collection of an accurately timed urine specimen is difficult for many patients.
24-Hour urinary free cortisol: The 24-hour urinary free cortisol test also requires accurately timed urine collections. Values in obese people rarely overlap with those in Cushing’s syndrome patients, and hepatic disease does not distort the result. The urinary free cortisol excretion rate has become a valuable index of adrenal hyperfunction. It is less reliable for the detection of hypoadrenalism.
Insulin-Induced Hypoglycemia ( = Insulin Tolerance Test)(ITT): An alternative method of measuring pituitary-adrenal reserve involves the IV administration of insulin (0.1 IU/kg). Blood is drawn at 30, 45, 60 and 90 minutes laters for serum cortisol and blood glucose. Positive if serum cortisol peak <18 ug/dL and if glucose decreased to <40 mg/dL (<2.22 mmol/L). Obese pt’s with insulin resistence should be given 0.15 IU/kg. Avoid in pt’s >60yo, those with h/o sz or CAD. The ensuing hypoglycemia should elicit both cortisol (normal, 20 g/dl) and growth hormone (> 8 ng/ml) responses. Abnormal is get little to no incr plasma cortisol, plasma BS <40mg/dL and plasma cortisol >20 mcg/dL. This test should be carried out in the presence of a physician prepared to administer 25 ml of 50% glucose IV in case of a hypoglycemic reaction.
In Primary Adrenal insufficiency: the basal plasma cortisol is low to low nl, will have plasma corticotropin >100pg/ml in primary adrenal insufficiency. Check Rapid ACTH Stimulation Test (Cortrosyn). If negative autoantibodies get CT/MRI of adrenals to r/o malignancy or TB, any mass need CT-Bx.
Conventional Corticotropin Test: get no incr in plasma cortisol n primary adrenal insufficiency. (nl range is basal or post-corticotropin >20 mcg/dL).
Other: if postmenopausal female, the FSH is expected to very elevated if normal pituitary function. Anti-adrenal Ab’s may help dx autoimmune adrenalitis. CT imaging of the adrenals may reveal hemorrhages, mets or infectious diseases. Adrenal bx is occasionally needed.
Laboratory findings in Adrenal Insufficiency:
Primary Adrenal Insufficiency:
1. Decreased cortisol
2. Increased CRH
3. Increased ACTH
Secondary Adrenal Insufficiency from Pituitary Etiology:
1. Decreased or Inappropirately Normal ACTH
2. Decreased Cortisol
3. Increased CRH
Secondary Adrenal Insufficiency from Hypothalamic Etiology:
1. Decreased CRH
2. Decreased or Inappropriately Normal ACTH
3. Decreased Cortisol
Tx: Chronic tx, see below.
Acute Adrenal/ Addisonian Crisis: 20ml/kg NS bolus to correct shock, then D25W 2ml/kg IV (0.5-1L/hr X 3-6hr) in some cases, blood should be administered to restore circulating volume. Abx if suspect sepsis (Rocephin 50mg/kg).
• If have a choice, use dexamethasone rather than hydrocortisone otherwise can’t work-up (ACTH-stimulation test) until out of system for days rather than a single day.
Hydrocortisone (Solu-Cortef): 1-2mg/kg IV (~100mg IV bolus then 100mg q8hr IV) X24hr. The tx of an adrenal crisis theoretically would require administration of ~200 mg of hydrocortisone daily. The standard regimen used in most centers provides 400 mg per day IV, ~ twice the amount calculated to be necessary. Dose can also be given at 50- to 100-mg doses I.M. or are diluted with dextrose in saline solution and given I.V. until the patient’s condition stabilizes; up to 300 mg/day of hydrocortisone and 3 to 5 L of I.V. saline solution are required during the acute stage of adrenal crisis. Other: Investigate and treat precipitants of adrenal crisis. Confirm diagnosis of adrenal insufficiency, if necessary. If possible, taper steroids to maintenance dose over 1–3 days. Start fludrocortisone 0.1 mg orally daily for patients with primary AI when saline infusion is discontinued.
DOCA (Deoxycortisol acetate): 1-2mg IM. Draw blood and store samples for later steroid level analysis. Beware of heart failure. If not improved in 4-6hr, reconsider dx. Check an ACTH stim test in 48hr. If the pt’s condition is improving, steroid doses can be tapered over three to five days to replacement levels.
Chronic AI Tx:
Wear Medic Alert-type bracelet. Replace the missing hormones. Aim is to establish and administer the lowest possible replacement dosage. Low blood pressure, sx’s of dizziness and excessive salt cravings or increased licorice intake indicate inadequate replacement therapy. Conversely, over substitution with an excess of glucocorticoids will cause weight gain and, sometimes, the clinical features of Cushing’s disease. Body-weight in particular should therefore be carefully monitored. Moreover, because excessive doses of glucocorticoid increase the long term risk of developing osteoporosis, regular bone mineral density measurements are required. Most with primary AI are also hypoaldosteronemia and need adequate salt intake and mineralocorticoids.
#1: Glucocorticoid replacement: Goal is to give the smallest dose that relieves the sx’s. Can check a 24hr urine-free cortisol & an 8AM plasma cortisol to fine tune. Can use HC, prednisone or dexamethasone. Individualized doses based on weight, age, pt’s sense of well-being, normalcy of BP/ temp/ HR, elimination of sx’s (anorexia, N/V, dizzy) and if on other meds (need more if pt taking phenytoin, rifampin, barbiturates, mitotane or aminoglutethimide). Can check ACTH to r/o noncompliance.
Patient Education on Avoiding adrenal crisis: A patient with adrenal hypofunction requires lifelong corticosteroid therapy. One needs to recognize the symptoms of too great (swelling, no sleep) or too little (weak, dizzy standing….) a dose. Tell the patient that the dose may need to be increased during times of stress (when he has a cold, for example). Explain that they need to increase oral glucocorticoids (2-fold to 4-fold) with serious illness, and to receive systemic therapy if they are vomiting. Medic Alert or SOS bracelets/pendants should be worn. The definition of stress is vague, but conservative criteria include fever >38°C (100°F), surgical procedures or injuries, and gastroenteritis with associated vomiting and diarrhea. It may be helpful to teach the patient how to give himself an injection of hydrocortisone. Advise the patient to keep an emergency kit available containing hydrocortisone in a prepared syringe for use in times of stress. Warn that any stress may require additional cortisone to prevent adrenal crisis. Warn that infection, injury, or profuse sweating in hot weather may precipitate adrenal crisis. Instruct the patient to always carry a medical identification card stating that he takes a steroid and giving the name of the drug and the dosage. Links: Stress Dose |
Hydrocortisone (Hydrocortone = HC, Cortef): [20mg tab, 10mg/5ml elixir] Most need 20-30mg divided in 2-3 doses. Usually can give BID. Start @ 15mg upon awakening, then 10mg @ 4-5PM. If still have afternoon fatigue give 15mg in AM, 5mg lunch and 5mg at 5PM. Use the smallest dose that improves sx’s. Max 80mg PO QID acutely. The classic dose is 20mg in AM and 10mg in the afternoon. Give physiologic replacement, ideally the therapy should be divided into 3 or 4 doses daily to mimic the natural variation of plasma hydrocortisone levels and to increase the general well being of the pt. If necessary, a more satisfactory response may be obtained by splitting the total daily dose of hydrocortisone into smaller doses and spreading them over shorter intervals. Even then, some pt’s will have low morning plasma levels of substituted cortisone and therefore feel tired in the morning. This can often be avoided by taking a single dose of hydrocortisone at 0400 hours, thus mimicking the natural diurnal levels of this hormone. HC is preferred because of its short half-life mimics most closely the normal cortisol circadian rhythm. The downside is that it must be given at least 2-3 times a day. Hydrocortisone also has some mineralocorticoid effects, which are useful if primary adrenal insufficiency.
Longer acting meds with a longer T-1/2 to avoid the peaks and valleys:
Dexamethasone (Deltasone, Hexadrol): 0.25-0.5 mg (=2.5-7.5mg Deltasone) qHS is sx’s already occurring upon awakening and/or hyperpigmentation from ACTH not resolving with hydrocortisone tx.
Prednisone: 2.5-5mg and 7.5mg.
#2: Mineralocorticoids: To prevent postural hypotension and maintain Na & K balance. Optimize glucocorticoid dose first as HCT has some mineralocorticoid effects. Needed for pt’s with primary AI.
Fludrocortisone (Florinef): [0.1mg, $27/#30 tabs] dose range needed is 0.05-0.2mg PO qAM or 0.1mg PO 3x/wk. Aim to keep plasma renin level at upper limits or normal. Adjust dose based on serum Na, K and postural hypotension. If excessive will get HTN, edema and decr K. Therapy should be titrated against the degree of salt craving and the magnitude of postural changes in blood pressure. During warm weather or excessive exercise, the fludrocortisone dose may need to be temporarily increased by an additional 0.05 to 0.1 mg/day. Marked increases in mineralocorticoid doses may also be necessary in pregnant women. All pt’s should be advised to modulate salt intake to their liking. The production rate of aldosterone is ~100 µg per day at all stages of life in salt-replete humans.48 Fludrocortisone is roughly equipotent with aldosterone but is available only as an oral preparation. In normal persons, mineralocorticoid activity is supplied by both aldosterone and cortisol in roughly equal proportions. Thus, if cortisol is used for replacement, fludrocor-tisone, 100 µg per day, will supply the remaining complement of mineralocorticoid activity.
#3: Give additional glucocorticoids under stress/ infection conditions. See Stress Dose | Typically double the maintenance dose.
Pregnant women should continue their basal supplementation but may need incr doses of glucocorticoid in the third tri. Women with hyperemesis gravidarum may not absorb adequate amounts of oral hydrocortisone and may therefore require IM injections. During labor, IV hydrocortisone @100mg should be given 6-hourly with continuous IV normal saline solutions and an extra dose of hydrocortisone 100mg administered just before the second stage of labor.
Other: Androgens can improve well-being and sexuality. Low-dose glucocorticoids used to treat primary adrenal insufficiency or congenital adrenal hyperplasia do not significantly alter bone mineral density (BMD) (J Clin Endocrinol Metab 2011;October 12th online).
Dehydroepiandrosterone (DHEA): 50 mg qd replacement therapy improves general health perception, arousal, and learning efficiency in hypoadrenal women (Endocrine Society 2004: Abstract P2-572. Presented June 17, 2004). Tx with DHEA significantly increases insulin sensitivity in hypoadrenal women and may prevent the onset of type 2 diabetes (Diabetes 2005;54:765-769).
Overnight IV administration of hydrocortisone in pt’s with adrenal insufficiency mimics the circadian rhythm and improves ACTH control (American College of Clinical Pharmacology. Abstract 114. Sept 12, 2005). In the traditional tx group, cortisol levels were very low in the morning, which makes pt’s feel tired in the mornings and makes it difficult to wake up…a delayed onset, slow release tablet administered before bedtime may have similar benefits.
Fidelin (Dehydroepiandrosterone / prasterone / DHEA): for adrenal insufficiency, has orphan drug designation from FDA.
Depression: The suppression of salivary cortisol levels by prednisolone provides a physiological assessment of the status of the hypothalamic-pituitary-adrenal (HPA) negative-feedback axis through significant differences between control subjects and pt’s with tx-resistant depression (19th Congress of the European College of Neuropsychopharmacology. Abstract P.2.a.003. Sept 19, 2006). Pt’s with TRD have reduced glucocorticoid receptor (GR) function and a hyperactive HPA, leading to high cortisol levels. This led to the use of the dexamethasone test to investigate the HPA axis status in these depressed pt’s. However, dexamethasone has pharmacodynamics and pharmacokinetics that are distinct from those of the endogenous glucocorticoids, and binds only to the GR. In contrast, cortisol binds to both the GR and the mineralocorticoid receptor…..prednisolone is a better corticosteroid than dexamethasone to assess the HPA axis because it binds to 2 different receptors, not only the glucocorticoid receptors but also the mineralocorticoid receptors, providing a more physiological approach.
Schmidt syndrome: Adrenal insufficiency + hypothyroidism. Replace glucocorticoids (at least one dose) before starting on thyroxine replacement. If not, the patient may die. When hypothyroid they can’t get to stressed, but if get thyroid active they can then get a stress response.
**Ref: (Adrenal d/o’s. A primary care approach. Lippincotts Prim Care Pract. 1997;1:527-36) (Williams Textbook of Endocrinology, 9th ed., 1998, WB Saunders) (Primary aldosteronism. NEJM 1998;339:25) (Eighty-six cases of Addison’s Disease. Clin Endocrinol (Oxf) 1994;41:757-61) (Diagnosis and management of adrenal incidentaloma. J of Urol 2000;163) (Autoimmune adrenal insufficiency: recognition and management. Bio Drugs 2000;13:107-14) (Corticosteroid supplementation in adrenal insufficiency. JAMA 2002;287:2) (Adrenal Insufficiency. JAMA. 2005;294:2481-2488)
For pt’s with known hypopituitarism, stress-dose steroids are crucial. Pt’s who have taken glucocorticoids for <3 weeks or who are on chronic alternate-day therapy should continue their usual dosage, since they are unlikely to have a suppressed hypothalamic-pituitary-adrenal axis. Pt’s believed to be at increased risk in this regard include those currently taking a pharmacologic dose of corticosteroid (>20-30 mg of hydrocortisone (HCT) daily, >5 mg of prednisone), those who have taken such doses for >2 wks in the preceding year, and those who are receiving replacement corticosteroid therapy for known adrenal insufficiency. Such pt’s undergoing major surgical procedures should receive their usual steroid dose by mouth the day before surgery. Glucocorticoids are produced in the adrenal cortex under the feedback control of both the hypothalamus and pituitary gland (hypothalamic-pituitary-adrenal [HPA] axis). The rate of cortisol secretion is equivalent to 20-30 mg/day of hydrocortisone or 5-7 mg/day of prednisone. This basal rate increases under conditions of severe stress, and the increase is essential for the maintenance of homeostasis.
Pt’s with chronic adrenal insufficiency (AI): Pt may need to double or triple their usual dose temporarily (up to ~3d w/o supervision) during a febrile illness. If severe N/V then come in for parenteral doses. Give 4mg prefilled Dexamethasone syringe if difficulty getting to medical care quickly. Pt’s should also be instructed to temporarily incr the dose of hydrocortisone if fever, infection or trauma develop. A general rule of thumb is to double the basal dose of hydrocortisone when the temperature is elevated above 38 deg C, and triple the basal dose when the temperature exceeds 39C. No gradual tapering of the dose is necessary after the fever has subsided. If there is vomiting and/or diarrhea, IV hydrocortisone may be required. Pt’s undergoing surgery should be given hydrocortisone 50-100mg IV q6 hrs. During elective surgery pt will general need 100mg IM/IV HCT q2hr before and 50-100mg q6hr after surgery for 24hr, then taper over 3-5d with resumption of Florinef when taking PO’s.
Dose: Typically 100mg Hydrocortisone IV q8hr. Can be given as Hydrocortisone 20 mg PO q6hr. If see severe stress give double or triple usual oral replacement dose and taper back to baseline as soon as possible.
Maintenance replacement: 10-20mg qAM and 5-10mg PO qPM.
Intravenous Hydrocortisone Dose Based on Procedure Risk
Minor: Preoperative: None. Postoperative: None.
Moderate: Preoperative:50mg. Postoperative:25mg q8h x 1–2 days.
Major: Preoperative:100mg. Postoperative:50mg q8h x 2–3 days.
Example of Surgical Dose with Taper:
Day #1: HCT 100 mg IV q8 starting with induction of anesthesia. First dose typically given in the early morning, with a second 100-mg dose administered intraoperatively. Postoperatively, 100 mg is given IV q8h for 24 hours
Day #2: if stable and major stress resolved, lower dose to 50mg IV q8hr.
Day #3: 25mg IV q8.
Day #4: 25mg IV q12h. At this point, the pt’s usual daily dose can often be reinstituted.
Day #5: Maintenance dose: 12-15mg HCT/m2/d or 15-20mg AM & 5-10mg PM.
If chronic adrenal insufficiency:
Minor Stress / Procedure / illness: Includes an inguinal hernia repair, colonoscopy, EGD, mild febrile illness, mod-mod N/V, gastroenteritis. 25mg HCT or 5mg IV methylprednisolone (MP) IV on the day of the procedure only. In pt’s undergoing relatively minor procedures (i.e., surgery of the distal extremities or those requiring only regional or local anesthesia), either the usual dose in a sip of water or a single preoperative dose of 100 mg of hydrocortisone IV is sufficient coverage. For very minor procedures, take maintenance dose only.
Outpatient management: use two or three times maintenance dose during illness, then return to maintenance dose. If illness does not resolve in 3 days, contact physician. Consider IV glucocorticoids (methylprednisolone) in patients who cannot tolerate oral medications.
Moderate Stress: Includes open choley, hemicolectomy, significant febrile illness, pneumonia, severe gastroenteritis. Moderately Stressful Procedures include endoscopy, barium enema, arteriography. 50-75mg Hydrocortisone (HC) PO for 2 days And 20mg q 8 hrs for 2 days. or IV twice a day. Or 10-15mg MP IV on day of procedure with taper over 1-2 days to usual dose.
Major Surgery: Hydrocortisone 100 mg IV just before induction of anesthesia. Continue hydrocortisone 100 mg IV every 8 hr for the first 24 hr. If no complications occur, taper by 50% per day to maintenance dosage.
Severe Stress: Includes major cardiothoracic surgery, Whipple procedure, liver resection, pancreatitis. 100-150mg HC or 20-30mg MP IV on day of procedure, then rapid taper over 1-2 days. Adjust dose according to patient’s condition. Taper rapidly over 1–2 days to maintenance dose after patient recovers.
Critically Ill: Includes sepsis-induced hypotension or shock. 50-100mg HC q6hr or 0.18mg/gk/h as a continuous infusion + 50 mcg/d of Fludrocortisone until shock resolved. May take several days to a week or more. Then gradually taper following vital signs and serum Na. (JAMA 2002;287:2)
Precautions: Patients should wear a medic-alert bracelet. Patients should carry syringes with 4 mg of dexamethasone in 1 mL of saline.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








