Key Clinical Questions
Why is it important to understand the regulation of normal sleep?
What are the most common causes of disrupted sleep in hospitalized patients?
What are the consequences of sleep deprivation?
What are the key questions that an examiner should ask when approaching a patient with impaired sleep?
What are nonpharmacologic and pharmacologic modalities for preventing and treating sleep problems in hospitalized patients?
A 59-year-old retired school teacher with a history of advanced ovarian cancer has been admitted for community-acquired pneumonia, responding well to appropriate antibiotic treatment. She has lost 10 pounds since her last clinic visit, and she reveals that she is having a difficult time sleeping during the night. Her husband is concerned that she has been “pretty down” recently. How should this problem be addressed? |
A 48-year-old woman with obesity, hypertension, depression, and asthma, was admitted to the hospital for treatment of an exacerbation of her multiple sclerosis. Her physicians continued her home medications including lisinopril, hydrochlorothiazide, bupropion, and interferon beta. After 48 hours, her weakness improved in response to intravenous high-dose methylprednisolone, but the doctor on call has been called every night for a “sleeper.” The patient stated: “I cannot sleep at all! I feel worse than when I came in! I just feel so anxious; this happens every time I have a flare.” She has also been having trouble staying asleep because she needs to get up multiple times each night to urinate, a nurse interrupted her sleep at 2 am every night to take her blood pressure, and her roommate has been watching TV all night. Although she uses continuous positive airway pressure (CPAP) at home, she forgot to bring the machine to the hospital. What adjustments can be made to improve the sleep of patients like her? |
Introduction
Sleep disruption is a common problem among hospitalized patients. Patients frequently report disturbed sleep not only during their hospital stay but also prior to and after discharge. Approximately one-third of hospitalized patients have insomnia at the time of admission. Additionally, up to 69% of postsurgical patients continue to complain of prolonged sleep problems after hospital discharge. The high prevalence of sleep disturbance among this population warrants clinicians to incorporate the evaluation and treatment of sleep problems as part of routine hospital care. In order to effectively treat sleep complaints, one has to understand the regulation of normal sleep and how various disorders may influence or impair this process. Early recognition and treatment of sleep complaints can improve recovery among hospitalized patients.
|
Pathophysiology
Even in healthy individuals, partial and total sleep deprivation significantly impacts sleep architecture and sleep quality. Evidence supports that persistent lack of sleep can impair physiologic processes and could potentially affect recovery from acute illness or injury. Sleep duration, architecture, and the sleep–wake cycle are closely associated with many metabolic and regulatory processes. Sleep deprivation can result in detrimental physiologic and psychological sequelae. Sleep deprivation has been associated with insulin resistance, impaired postural control, decreased ventilatory drive, increased sympathetic cardiovascular activation, blunted hypothalamic–pituitary–adrenal axis responsiveness, and impaired host defenses. The lack of restorative sleep increases the risk of developing anxiety, mood disorders, and delirium, especially in acutely ill older patients. In the presence of acute physical infirmity, inadequate sleep may further compound illness and recovery.
Sleep architecture refers to a characteristic pattern of sleep and includes two major stages: non–rapid eye movement (NREM) and rapid eye movement (REM) sleep. Normal sleep latency, or time to fall asleep, is usually 10 to 20 minutes, and total sleep time ranges from 6 to 9 hours per 24 hours. NREM sleep consists of three stages (S1–S3), with each stage leading to a progressively deeper level of sleep. S3 is known as deep sleep or slow-wave sleep (SWS), is predominant during the first third of the sleep period, and is believed to be necessary for physiologic restoration. SWS is associated with a decrease in metabolic rate, heart rate, and oxygen consumption and is anabolic in protein and hormone synthesis. REM sleep follows SWS and is characterized by an activated brainwave pattern seen with electroencephalography (EEG), muscle paralysis (atonia), and periodic bursts of rapid eye movements. Dreaming occurs during REM sleep and is hypothesized to be essential for emotional and cognitive well-being. REM sleep is a catabolic phase and can be associated with cardiovascular and respiratory instability. REM sleep occurs predominantly in the later period of sleep. Additionally, the ability to consolidate sleep declines with age, requiring most elderly persons to nap during the day in order to achieve a normal total amount of sleep.
Sleep disruption may also occur when regulatory processes are impaired or altered. Sleep regulation is a balance between a homeostatic or biologic need for sleep or “sleep debt” and the intrinsic body clock, or circadian pacemaker. Homeostatic mechanisms are regulated in the preoptic area in the brain, while circadian systems are governed by the suprachiasmatic nucleus (SCN) of the hypothalamus. Melatonin, a hormone produced by the pineal gland, is associated with sleep induction. It builds up as darkness increases and is inhibited by ambient light. The adrenal secretion of cortisol follows a circadian pattern and peaks in the early morning hours in preparation for the increased metabolic demands during wakefulness. Neurotransmitters are intrinsically involved in sleep regulation and include gamma-aminobutyric acid (GABA), serotonin, histamine, norepinephrine, acetylcholine, hypocretin, glutamate, and glycine. REM sleep is promoted by acetylcholine. SWS is induced primarily by GABA. Neurotransmitters that promote wakefulness include acetylcholine, histamine, noradrenaline (norepinephrine), serotonin, dopamine, and hypocretin (orexin). Many of the drugs that are commonly prescribed in hospitalized patients affect one or more of these neurotransmitters, thus promoting sedation (antihistamine) or activating effects (dopamine) such as wakefulness, and, in vulnerable patients, delirium.
Etiology and Differential Diagnosis of Sleep Complaints
There are numerous factors that may contribute to disturbed sleep in hospitalized patients, including primary sleep disorders, medical illnesses, psychiatric illness, drugs, and the hospital environment. Additionally, the manifestations of sleep disruption and/or sleep deprivation will vary depending on the individual.
By far, insomnia is the most common sleep complaint among patients in both ambulatory and hospital settings. The prevalence of chronic insomnia is high, with approximately 20–30% of the general population reporting ongoing symptoms. Chronic insomnia is associated with decreased quality of life, daytime functional limitations, chronic pain, increased risk of medical and psychiatric illnesses, substance abuse, increased utilization of health services, and increased risk of death.
Insomnia can manifest in several ways. The International Classification of Sleep Disorders (ICSD-2) published by the American Academy of Sleep Medicine (AASM) defines insomnia as difficulty initiating or maintaining sleep, waking up too early, or sleep that is chronically nonrestorative or perceived to be poor in quality. To meet diagnostic criteria for insomnia, these symptoms must be associated with daytime mental or physical sequelae that impair the functional status of the individual. Insomnia may be a primary disorder or may be comorbid with another physical or mental illness.
In the landmark Sleep Heart Health Study, population subgroups were identified who were at increased risk for poor sleep. Older individuals (> 60 years old) were shown to have a lower arousal threshold associated with increased awakenings at night, decreased sleep efficiency, and decreased REM quantity. Each of these changes contributes to poor sleep quality and can result in poor daytime functioning, excessive sleepiness, or cognitive changes, which are commonly noted in the elderly. Although women demonstrated increased SWS compared to men, they had more complaints of poor sleep quality associated with worsening daytime functioning. The precise reason for this is unknown but is speculated to involve hormonal influence as well as greater prevalence of comorbid conditions associated with insomnia, such as depression.
Other common primary sleep disorders that frequently coexist with medical illnesses include obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movement disorder (PLMD). Table 94-1 describes these common sleep disorders and provides differential diagnoses to consider. These disorders may present with a variety of symptoms.
| Sleep Disorder | Clinical Features | Differential Diagnosis |
|---|---|---|
| Obstructive sleep apnea (OSA) | Repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation. Episodes include loud snoring or gasps lasting 20–30 seconds. Associated with morning headaches and dry mouth. | Sleep-related laryngospasm, nocturnal gastroesophageal reflux, narcolepsy, hypersomnia, PLMD, central alveolar hypoventilation, paroxysmal nocturnal dyspnea, primary snoring, Cheyne-Stokes ventilation, nocturnal asthma. |
| Periodic limb movement disorder (PLMD) | Periodic episodes of repetitive and stereotyped limb movements: extension of the big toe with partial flexion of the ankles, knees, or hips. Muscle contractions last 0.5 to 5 seconds, with 20- to 40-second intervals between them. | Sleep starts (occurs just prior to, not during, sleep, and does not have a regular periodicity like PLMD), nocturnal epileptic seizures, myoclonic epilepsy. |
| Restless leg syndrome (RLS) | Uncomfortable leg sensations that occur prior to sleep onset that leads to an irresistible urge to move the legs. Described as “achy,” “crawling,” “pulling,” “prickling,” or “tingling,” and disrupts sleep onset. | Chronic myelopathy, peripheral neuropathy, akathisia, fasciculation syndromes. RLS may be triggred by iron deficiency anemia, so consider iron studies. |
| Sleep starts | Sudden, brief contraction of the legs that occurs at sleep onset. Usually benign, but may worsen during hospitalization, and interfere with sleep. | PLMD, RLS, hyperexplexia syndrome in which generalized myoclonus is readily elicited by stimuli. |
OSA affects approximately 24% of men and 9% of women in the United States and is associated with substantial mental and physical morbidity. Untreated OSA likely contributes to a significant proportion of acute hospital admissions, particularly for heart or respiratory failure. OSA may go unrecognized until a patient is incidentally hospitalized and observed to have difficulty breathing, paradoxical breathing, or oxygen desaturation. Risk factors for OSA include obesity, hypothyroidism, male gender, family history, African American race, and certain craniofacial characteristics: acromegaly, micrognathia or retrognathia, large tongue, or Mallampati class 3–4. OSA is characterized by episodes of complete or partial pharyngeal obstruction during sleep that cause snoring, apneic episodes, choking, dyspnea, and restlessness. These episodes are associated with intermittent nocturnal sympathetic activation leading to nocturnal awakenings and cortical arousals, all of which lead to daytime symptoms of fatigue, sleepiness, and cognitive impairment. Chronic sympathetic activation and intermittent hypoxemia are associated with increased vascular injury and inflammation that are known to occur in cardiovascular disease. Treatment of OSA with positive airway pressure (PAP) is shown to improve control of hypertension, diabetes, pulmonary hypertension, atrial fibrillation, and mortality.
Approximately 20% of patients with OSA will have concomitant RLS or PLMS/D, which are distinct problems and need to be differentiated from peripheral neuropathy and positional or nocturnal leg cramps. RLS is thought to affect as much as 40% of the population and is characterized by an unpleasant crampy, “creeping” or “crawling” sensation in the lower extremities that is relieved by persistently moving the legs. RLS frequently starts in the late evening or before bedtime, and often is a major cause of sleep-onset insomnia. The requisite bed rest during hospitalization can worsen RLS, further exacerbating sleep problems. It is also associated with many metabolic disorders, particularly renal disease, iron deficiency, and diabetes. Other conditions associated with RLS include pregnancy, rheumatoid arthritis, fibromyalgia, multiple sclerosis, and Parkinson disease. The etiology of RLS is not completely understood, but it may relate to inadequate generation or transport of dopamine due to iron deficiency or other metabolic disturbances. Serum iron and ferritin levels should be evaluated and treated if ferritin levels are < 75 μg. Aggressive treatment of other underlying diseases should be considered as first-line therapy. Selective serotonin uptake inhibitors (SSRIs) and alcohol may exacerbate RLS and should be avoided. Additional treatment includes the use of a dopaminergic agent at bedtime, such as ropinirole (Requip). Other agents that are effective, particularly in individuals with neuropathy, are gabapentin, narcotics, and benzodiazepines.
PLMD occurs in about 80% of those with RLS and is characterized by stereotyped involuntary limb movements that occur every 20 to 40 seconds during sleep. These can result in frequent cortical arousals, daytime somnolence, and fatigue. PLMS are found at higher frequency in several medical conditions, including hypertension, renal disease, and alcohol dependence. PLMS can be treated with longer-acting benzodiazepines such as clonazepam, and with dopaminergic agents.
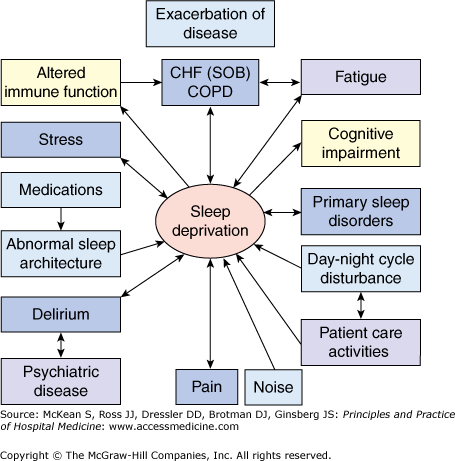
Numerous medical illnesses can directly impair sleep physiology, leading to a cyclical interaction in which impaired sleep impedes recovery (Figure 94-1). Table 94-2 lists selected medical and neurologic conditions, their associated sleep-related problems, and suggestions on how to alleviate these problems.
| Disease | Effect on Sleep | Interventions to Improve Sleep |
|---|---|---|
| CHF | Orthopnea, paroxysmal nocturnal dyspnea, sleep-disordered breathing, increased sympathetic tone, nighttime diuresis, Cheyne-Stokes respiration. | Keep the head of bed elevated ≥ 30 degrees. Nocturnal O2 to keep O2 saturation > 88%. Daytime diuresis. Optimize cardiac function to treat Cheyne-Stokes respiration. Consider CPAP for CHF. |
| COPD | Persistent nocturnal hypoxemia with complications (eg, cor pulmonale, polycythemia). | O2 for COPD and persistent hypoxemia (PaO2 55–60 mm Hg). |
| Sporadic nighttime desaturations. | PaO2 ≤ 55 mm Hg → monitor O2 saturation by pulse oximetry. If patient desaturates to ≤ 88% at night consistently, start nocturnal O2. For hypercapnea, adjust O2 to maintain O2 saturation at 88–90%. | |
| Increased risk of airflow obstruction during REM. | Consider bedtime tiotropium and inhaled long-acting beta-adrenergic agonist agents. | |
| Inhibition of respiratory muscles in REM. | Avoid sedative-hypnotics that cause respiratory depression. | |
| Decreased functional residual capacity from recumbent position during sleep. | Keep the head of bed elevated > 30 degrees. | |
| ESRD | Pruritus, nausea; increased risk of RLS and PLMD in patients with ESRD. | Consider ropinirole and pramipexole if RLS; if medications contraindicated, massage or walking will relieve discomfort. Correct hyperphosphatemia and uremia. Consider antipruritic and antiemetic agents. |
| Thyroid Disorders | Hypothyroidism—daytime hypersomnolence. | Treat underlying thyroid dysfunction. Discourage naps during the day, keep lights on during the day, and off during the night. |
| Hyperthyroidism—hyperarousal symptoms (restlessness, tachycardia, diaphoresis, anxiety). | Treat underlying thyroid dysfunction. Low-dose beta-blocker (eg, propranolol, atenolol) for symptomatic relief. | |
| Diabetes | Bedtime hyperglycemia → polydipsia, polyuria → frequent awakenings; or unnecessary nighttime monitoring of glucose levels. Inadequate scheduled or as needed insulin doses? High carbohydrate intake at bedtime? | Optimize blood glucose control. Increase scheduled or as needed insulin doses; consider consulting a dietitian to assess actual carbohydrate intake in the evening. Once bedtime glucose levels consistently ≤ 200 mg/dL, and no overnight hypoglycemic episodes, consider decreasing frequency of glucose monitoring at night. |
| Early morning (eg, 0200–0400) hypoglycemic episodes → symptoms awaken patient, or lead to frequent routine glucose monitoring overnight. | Optimize blood glucose control. Once bedtime glucose levels consistently ≤ 200 mg/dL, and no overnight hypoglycemic episodes, consider decreasing frequency of glucose monitoring at night (eg, if currently checking every 2 h → every 4 h or more appropriate?) | |
| Stroke | Focal neurologic deficits (eg, dysphagia, weakness or paralysis). | Keep head of bed > 30 degrees. Regularly suction secretions. Poststroke patients have an increased risk of hypersomnia, insomnia, and/or OSA. |
A recent study examined risk factors for sleep disturbance during hospitalization and found that the severity of comorbid conditions and poor performance of activities of daily living (ADL) predicted sleep complaints during admission. Physician awareness of the impact of sleep disturbance in hospitalized patients is vital since about half of patients admitted on general medical wards will complain of sleep disruption.
“Red flag”
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree