CHAPTER 23
Inflammatory Bowel Disease
Catherine M. Concert, DNP, RN, FNP-BC, AOCNP, NE-BC, CGRN
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic inflammatory diseases of the bowel and share many demographic, epidemiologic, and clinical features. Because UC and CD lack any unique distinguishing features and can resemble many other diseases, there is considerable potential for misdiagnosis. The essential nature and etiology of inflammatory bowel diseases are unknown; however, understanding of the clinical patterns, immune dysfunction, environmental factors, and genetic predisposition underlying these conditions has increased considerably and has spurred the development of new therapies. The early recognition of UC and CD and appropriate management can spare patients hospitalization and surgery, while improving quality of life. Primary care providers are invaluable members of the team caring for patients with inflammatory bowel disease (IBD) and can claim a leading role in early disease recognition and diagnosis, coordination of management among multiple specialties, prevention of disease recurrence, and cancer screening.
 ANATOMY, PHYSIOLOGY, AND PATHOLOGY
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
Intensive investigation over the past 75 years has failed to produce a simple explanation of the pathophysiology of IBD. IBD is known as an idiopathic disease. Current research focuses on identifying genes associated with IBD, and the interaction of bacteria in the gastrointestinal tract with the immune system (Denson et al., 2013). An infectious agent has eluded investigators, and searches for multiple candidate parasites, mycobacteria, viruses, and bacteria have proved futile. There is strong evidence that immune cell dysfunction, especially T-cell activation (Th1, Th2, and Th17), plays an important role in UC and CD (Khor, Gardet, & Xavier, 2011; Monteleone, Sarra, Pallone, & Monteleone, 2012). Activated T lymphocytes produce interleukin-2 (IL-2; an inflammatory cytokine), which may play a role in the inflammatory cascade in which the activation of other T cells, B cells, and macrophages occurs. Macrophages, the first line of defense, present luminal antigens to the sensitized T cells and release a host of proinflammatory cytokines. The cytokines produced in the cascade amplify the inflammatory response by recruiting neutrophils and monocytes. The subsequent release of oxygen metabolites, proteases, and other inflammatory cytokines then produces macroscopic mucosal injury (Bhan, 2012; Fiocchi, 2012; Hundorfean, Chiriac, Mudter, & Neurath, 2013; Sanchez-Muñoz, Dominguez-Lopez, & Yamamoto-Furusho, 2008). Tumor necrosis factor alpha (TNF-α) and other cytokines then perpetuate the inflammatory response (Tillack et al., 2014). The enteric nervous system and neuropeptides such as somatostatin may also play a role in regulating or perpetuating the inflammatory cascade. Platelet dysfunction and coagulation abnormalities, in close consort with the inflammatory cascade, are likely to contribute to the injury of the bowel.
Crohn’s Disease
CD is characterized by transmural granulomatous inflammation. It can involve any part of the gastrointestinal tract from the mouth to the anus, but most commonly involves the terminal ileum (Crohn, Ginsburg, & Oppenheimer, 1932). CD can also be called regional enteritis, and when it involves the colon, granulomatous colitis. Inflammation tends to be patchy and noncontiguous; a mucosal biopsy taken during endoscopy may miss submucosal involvement. Colonic involvement typically spares the rectum.
Early in the inflammatory process, edema, hyperemia, and aphthous ulceration of the mucosa predominate. As the disease progresses, these aphthae can enlarge and coalesce to form deep, serpiginous ulcerations with nodular swelling of the intervening inflamed mucosal lining, producing the classic cobblestone appearance seen on contrast radiography. The bowel can then become thickened, fibrotic, and narrowed. The surrounding mesentery can also become edematous and fatty and can even encase the involved bowel segment, producing a phlegmon. A phlegmon or abscess can produce an abdominal mass palpable on physical examination. Nerve fiber hyperplasia in the submucosa is common (Geboes, 2008; Yu, Wang, Zhao, Ma, & Jin, 2012). Fistulas, the result of transmural inflammation and fissuring, can penetrate the bowel wall, producing local perforation and abscess formation. These fistulas can communicate with adjacent bowel, bladder, vagina, or even skin, especially around the anus.
Ulcerative Colitis
The inflammatory process of UC involves only the colonic mucosa layer. The inflammation can involve the rectum and sigmoid colon or the entire colon. UC is usually symmetrical and continuous and involves the colon from the anal verge. Some patients present with isolated proctitis that can progress to involve the proximal colon. If the disease involves the entire colon (also called universal colitis) and the inflammation is severe, indirect injury of the terminal ileum, called backwash ileitis, can occur. Otherwise, any proximal gut or small bowel involvement implies CD.
Early inflammation can produce hyperemia, edema, and friability. As the inflammatory process progresses, spontaneous hemorrhage and superficial ulcerations of the mucosa develop. These can become diffuse and coalesce, forming deep, confluent ulcerations. With chronic recurrent injury, fibrosis can develop. Pseudopolyps, the result of chronic inflammation and healing, can protrude into the colonic lumen and cause obstruction. Long-standing inflammation can cause stricture formation, which can be a harbinger of underlying adenocarcinoma in patients with UC.
Severe inflammation causes thinning and dilation of the bowel wall and denudement of the mucosal lining, compromising the protective mucosal barrier. Toxic dilation, also called megacolon, can occur and possibly lead to perforation. Small rectovaginal or perirectal fistulas are rare but can occur in UC.
These distinguishing features of CD and UC become less reliable with chronic or severe disease and after successful treatment. Healing of UC can be uneven or patchy. Use of rectal or topical medications can produce rectal sparing similar to Crohn’s colitis.
Pathology
An IBD diagnosis is not always proven with endoscopic biopsies alone; it requires careful clinicopathological correlation. An endoscopic biopsy can provide a correct diagnosis of CD in 64% and a UC diagnosis in 74% of patients (Geboes, 2008). The traditional histological, pathognomonic feature of CD, the noncaseating epithelioid granuloma, is found in only 10% to 28% of endoscopic biopsies and only half of surgical specimens. Histological features that favor CD include epithelioid granulomas, discontinuous crypt distortion, discontinuous inflammation, and focal cryptitis. Transmural mucosal inflammation, fissures, and skip lesions are also pathological features of CD (Brand, 2009; Joseph & Weber, 2012). The inflammatory process can traverse all four layers of the bowel, up to and including the serosal layer.
The typical histological feature of UC is the crypt abscess with proliferation of neutrophils in the lamina propria. A heavy and diffuse mucosal inflammatory infiltrate and severe mucin depletion are additional characteristics. Histological features that favor UC include severe distortion or atrophy of the crypts with a villous or irregular mucosal surface. These histological changes can even be seen in endoscopically normal-appearing mucosa (Geboes, 2008; Joseph & Weber, 2012).
 EPIDEMIOLOGY
EPIDEMIOLOGY
There is considerable geographic variation in the incidence of IBD. The incidence of IBD is increasing in the Western world, with higher rates in more developed countries in North America and Europe (Molodecky et al., 2012). The incidence of CD and UC is becoming a global disease burden (Burisch & Munkholm, 2013).
The highest annual incidence of UC in Europe is 24.3 per 100,000 person-years; in Asia and the Middle East the incidence is 6.3 per 100,000 person-years; and in North America it is 19.2 per 100,000 person-years. The highest annual incidence of CD in Europe is 12.7 per 100,000 person-years; in Asia and the Middle East the incidence is 5.0 per 100,000 person-years; and in North America it is 20.2 per 100,000 person-years. The highest prevalence values for IBD are in Europe. UC prevalence is 505 per 100,000 persons and CD prevalence is 322 per 100,000 persons. In North America the prevalence for UC is 249 per 100,000 persons and CD prevalence is 319 per 100,000 persons (Khalili et al., 2012; Molodecky et al., 2012).
Both are diseases of young people, and a diagnosis of IBD is most likely to be made in patients in their teens extending to 40 years of age. The highest incidence of IBD is in patients in their 20s (Ha, 2012). Men and women have a slight difference with female predominance in CD and male predominance in UC. Although these diseases can occur in any age group, a second peak in incidence can occur in 10% to 15% of patients in their seventh and eighth decades of life (del Val, 2011; Ha, 2012). This second peak may be attributable to confusion of diverticulitis or mesenteric ischemia with IBD or, more likely, a result of more intense evaluation of elderly patients suspected of having IBD.
Whites are more likely to have IBD than patients of Asian, African, and Hispanic descent. IBD is more common among patients of Jewish heritage. In Israel, IBD rates are higher among European- and American-born Jewish populations than those from Africa and Asia. The geographic variability in incidence among Jewish populations appears to mirror that of the general population. Migrants to areas of higher incidence subsequently exhibit a higher rate of IBD. Studies that have indicated a higher incidence among urban residents or among members of certain occupations are probably undermined by referral bias.
Genetic Factors
A genetic component is suspected in IBD. Although the concordance between monozygotic twins is significantly <100%, siblings of patients with IBD are 17 to 35 times more likely to have IBD than the general population. Approximately 10% to 25% of IBD patients have a first-degree relative with UC or CD. Progress for finding UC and CD genes has been promising, with more than 160 genes identified (Denson et al., 2013; van Schaik et al., 2013). It is known that the NOD2 gene on chromosome 16 is a marker for susceptibility to CD. The NOD2 gene is present in 4.4% of Hispanics and 9.1% of the White population. Gene discoveries specific to CD are associated with NOD2, IRGM, and ATG16L1. There is genetic evidence demonstrating that specific genes such as HNF4A, LAMB1, CDH1, and GNA12 are associated with UC.
Environmental Factors
Certain environmental factors contribute to the pathogenesis of IBD. Smoking is positively associated with CD, but in UC a negative association has been observed. There is slight increased risk for IBD among users of oral contraceptives (Cornish et al., 2008; Frolkis et al., 2013; Lakatos et al., 2013; Rosenfeld & Bressler, 2012).
Diet would seem a logical focus of investigation, but numerous studies examining diet and IBD have failed to demonstrate any dietary risk factors. Western-style diet (processed and fried food) and increased sugar intake are suggestive of an increased risk of CD. Low vegetable and fiber intake has been associated with development of IBD. A high intake of animal fats, polyunsaturated fatty acids, and milk protein can have an increased incidence of UC and CD (Andersen, Olsen, Carbonnel, Tjønneland, & Vogel, 2012; Yamamoto, 2013). There is no consistent evidence that prenatal vitamin supplements, tonsillectomy, childhood vaccinations, early childhood hygiene, obesity, toothpaste use, psychosocial factors, and breastfeeding or bottle-feeding play any role in the etiology of IBD (Long et al., 2012; López-Serrano, Pérez-Calle, & Fernández-Rodríguez, 2010; Shaw, Blanchard, & Bernstein, 2011).
 DIAGNOSTIC CRITERIA
DIAGNOSTIC CRITERIA
There are no set diagnostic criteria for IBD. The diagnosis of CD is based on the history and clinical profile and supporting radiographic, histological, or endoscopic data. Most patients will give a history of at least 6 weeks of symptoms, thus excluding most acute infectious enterocolitides. Several conditions can act as impostors of CD, including tuberculosis, Yersinia enteritis, Entamoeba histolytica, and Chlamydia. Appendicitis, intestinal lymphoma or carcinoma, carcinoid tumor of the small bowel, celiac sprue, and diverticulitis often can be mistaken for CD.
UC can be mistaken for several conditions that produce inflammation and ulceration of the colonic mucosa and bloody diarrhea: Escherichia coli, Salmonella, Shigella, Campylobacter, E. histolytica, and cytomegalovirus. Other impostors include diverticulitis, cancer of the colon, ischemic colitis, nonsteroidal anti-inflammatory drug colopathy, radiation injury to the rectum, pseudomembranous or antibiotic-associated colitis, and solitary rectal ulcer syndrome (Hisabe, Hirai, Matsui, & Watanabe, 2013; Hundorfean, et al., 2013; Quigley & Bernstein, 2012; Sonnenberg & Genta, 2012).
 HISTORY AND PHYSICAL EXAMINATION
HISTORY AND PHYSICAL EXAMINATION
Patients with IBD can present with multiple, often confusing symptoms. Certain patterns in the history of these patients allow the provider to distinguish IBD from other gastrointestinal diseases and between UC and CD (Table 23.1).
Crohn’s Disease
Clinical Features Differentiating Ulcerative Colitis From Crohn’s Disease |
ULCERATIVE COLITIS | CROHN’S DISEASE | |
Gross blood in stool (hematochezia) | Almost always | Occasionally |
Mucus | Almost always | Occasionally |
Systemic symptoms | Occasionally | Frequently |
Pain | Occasionally | Frequently |
Abdominal mass | Rarely | Frequently |
Significant perineal disease | No | Frequently |
Extraintestinal manifestations | Frequently | Frequently |
Fistulas | No | Frequently |
Abscess | No | Occasionally |
Intestinal obstruction | Rarely | Frequently |
Response to antibiotics | Occasionally | Frequently |
Recurrence after surgery | No | Frequently |
Current smoker | Rarely | Frequently |
Former smoker | Frequently | Rarely |
Previous appendectomy | Rarely | Occasionally (“missed”) |
Source: Baumgart and Sandborn (2007).
The patient with CD commonly presents with systemic symptoms, including malaise, fever, night sweats, and weight loss. Most commonly, patients complain of right lower quadrant pain indicative of distal ileal involvement. Involvement of the stomach and duodenum can produce pain similar to that of peptic ulcer disease. As the disease progresses, chronic scarring can cause gastric outlet or duodenal obstruction.
In the teenage patient, a history of developmental delay resulting from malabsorption may be elicited. Patients will often give a history of increased borborygmi. Nocturnal abdominal pain, severe enough to interrupt a sound sleep, or nocturnal bowel movements help to distinguish IBD from functional syndromes of the bowel. Gross rectal bleeding may be seen but is unusual. Most patients complain of frequent, loose, nonbloody stools and right lower quadrant pain.
Symptoms of intermittent small intestinal obstruction or a frank perforation may suggest underlying CD. Fistulas can penetrate adjoining abdominal or perineal structures such as the urinary bladder, producing pyuria, fecaluria, or pneumaturia. Penetration of the skin or vagina may present with passage of air, stool, or mucus. Complications, including toxic megacolon and colonic perforation, associated with UC can also be seen in CD. Patients may give a history of “missed” appendectomy—that is, ileitis that was mistaken for appendicitis.
Ulcerative Colitis
UC patients most often present with bloody stools accompanied by mucus and diarrhea. If only the rectum is inflamed, patients may complain of constipation with a sense of urgency and passage of bloody mucus. The passage of gross blood is the cardinal feature of UC. Disease limited to the distal or left colon is usually not accompanied by constitutional symptoms, whereas universal or more severe disease can produce symptoms of malaise, nausea, and diffuse abdominal pain. UC has a strong tendency toward recurrence, and patients may give a previous history of hospitalization for severe disease or toxic megacolon. A history of recent discontinuation of tobacco may also be elicited.
Extraintestinal manifestations occur in both UC and CD. The most common extraintestinal manifestations are musculoskeletal and mucocutaneous, including axial and peripheral arthritis, acute ocular inflammation, erythema nodosum, and pyoderma gangrenosum. The most significant musculoskeletal manifestation is ankylosing spondylitis, which can occur in 1% to 5% of patients. The arthritis affects the larger joints. Some manifestations may precede the bowel symptoms, and diagnosing IBD may be difficult. Other manifestations (e.g., ankylosing spondylitis) are not related to the severity of the disease, and treatment is challenging. Common extraintestinal manifestations of CD are anemia, amyloidosis, aphthous stomatitis, cholangitis, cholelithiasis, episcleritis, erythema nodosum, inflammatory arthropathies, nephrolithiasis, osteoporosis, and uveitis. Arthritis, oral ulcerations, primary sclerosing cholangitis, pericholangitis, and pyoderma gangrenosum are common extraintestinal manifestations of UC. Hypercoagulability (deep vein thrombosis, cerebrovascular accident, pulmonary embolus) is an extra intestinal manifestation that can be present in both (Veloso, 2011).
 DIAGNOSTIC STUDIES
DIAGNOSTIC STUDIES
An IBD diagnosis is formulated from a combination of histological, radiological, endoscopic, and/or biochemical surveillance, as a single gold standard is not available (Panes et al., 2013).
Laboratory Tests
IBD can produce many abnormalities in routine screening laboratory tests. Anemia, leukocytosis, and elevated platelet counts may be noted on the complete blood count. Patients with UC are more likely to have a microcytic anemia, whereas those with CD of the small bowel usually have macrocytic anemia from malabsorption of cobalamin (vitamin B12) or folic acid. The erythrocyte sedimentation rate and the C-reactive protein level, both nonspecific indicators of inflammation, can be elevated in both forms of IBD. Abnormal liver function tests, including levels of alkaline phosphatase and transaminases, may be seen in IBD because of reactive hepatitis or primary sclerosing cholangitis.
Low serum protein and albumin levels can be seen in IBD and can indicate chronicity and severity. Electrolyte abnormalities, including hypokalemia, hypomagnesemia, and hypocalcemia, can be documented in many IBD patients, especially those taking steroids.
Serologic blood tests to help distinguish between UC and CD are available. The presence of perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) suggests UC, with 70% of UC patients having high titer antibodies to p-ANCA and 20% of CD patients having low titers. The presence of high titers of anti-Saccharomyces cervisiae antibodies (ASCA) and anti–outer membrane protein C (OmpC) suggests CD (Vasseur et al., 2012; Zhang et al., 2012). Low titers of these can be seen in UC patients (Strober & Gottesman, 2009). Pancreatic autoantibodies (PAB) have been discovered to be specific for CD and goblet cell autoantibodies (GAB) have specificity for UC, but sensitivity is low for both tests (Prideaux, De Cruz, Ng, & Kamm, 2012; Tesija Kuna, 2013). However, 10% of patients with IBD may not be categorized by these assays.
Radiography
Once a diagnosis of IBD is suspected, contrast radiography is a useful tool in making the diagnosis of IBD but not in determining clinical activity.
CD most often affects the distal ileum and produces a characteristic appearance on a barium small bowel series. Stricturing, thickening of the bowel loops, fistulas, and aphthous ulceration can be detected in a small bowel series in a patient with CD. A normal small bowel series is usually seen in UC. Barium enema is useful in evaluating the extent of disease in UC and the presence of fistula in CD. It does not, however, correlate with disease severity and is not useful in screening for precancerous changes (Cotter, Christopher, Jadhav, Sengupta, & Keohane, 2013). Computed tomography (CT) and magnetic resonance imaging (MRI) are useful for patients who cannot undergo standard endoscopic evaluations. MRI is used for the diagnosis of CD (Bruining & Loftus, 2008). CT can evaluate bowel wall thickening or an abdominal mass of CD if an abscess is suspected. CT-guided needle aspiration of an intra-abdominal abscess associated with CD rarely prevents surgery. Ultrasonography can detect hydronephrosis associated with CD, but overall plays a lesser role in the evaluation and management of IBD (Bruining, 2012).
Endoscopy
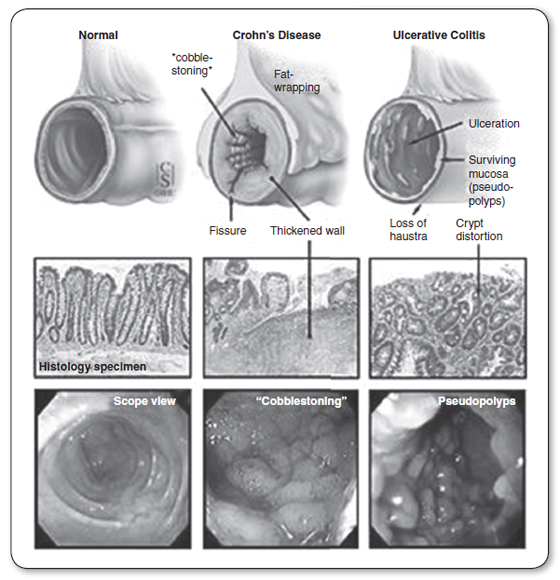
Endoscopy is regarded as the gold standard for diagnosis of IBD. Figure 23.1 illustrates endoscopic and histological views of IBD. Biopsy sampling during endoscopic examination can demonstrate histopathological features of UC such as confluent inflammatory cellular infiltration closest to the epithelial surface. In CD, patchy, deep, and focal inflammatory cell infiltrates and macrophages are seen that can form noncaseating granulomas containing giant cells (Strober & Gottesman, 2009). Flexible sigmoidoscopy or even proctoscopy, especially when used in conjunction with a small bowel series, may be used to confirm a diagnosis of IBD. These modalities are particularly useful in the patient with severe, active colitis, where colonoscopy should be avoided. Colonoscopy is useful when the diagnosis is in question, the colitis is more quiescent, or surveillance colonoscopy is needed. The rectal mucosa is almost always involved in UC, whereas in CD, the rectum is often spared. Video capsule endoscopy (a swallowed pill camera) can be used to evaluate the small bowel for ulcers in patients with CD (Moscandrew & Loftus, 2008).
FIGURE 23.1
Endoscopic and histological views of inflammatory bowel disease (IBD). These pictures demonstrate normal bowel viewed with an endoscope compared to endoscopic views of the bowel with Crohn’s disease and ulcerative colitis.
Source: Adapted from “Ulcerative Colitis-Introduction” by Johns Hopkins Medical Institutions. Copyright 2013 by Johns Hopkins Medical Institutions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree