Key Clinical Questions
What factors predispose to the development of IBD?
Which patients with IBD require hospital admission?
How can clinicians induce and maintain remission quickly and safely to prevent progression of disease and surgical intervention in hospitalized patients with inflammatory bowel disease (IBD)?
How can clinicians minimize adverse events related to IBD, its medications, and related surgery?
Introduction
Significant heterogeneity in the geographic distribution of inflammatory bowel disease (IBD) exists, but a north-south gradient has been confirmed in multiple studies. Both conditions, ulcerative colitis (UC) and Crohn disease (CD), are more common in commercially insured and individuals of the upper middle class than those covered by Medicaid. A total of approximately 1.5 million Americans are affected by UC and CD. In adults, the prevalence of UC and CD was 238 and 201 per 100,000 people, respectively. The incidences for UC and CD range from 7.6–8.8 and 6.9–7.9 per 100,000 people, respectively. In developed countries, incidence rates have been increasing for CD, but are stable for UC. Further, in comparison to the general population, patients with IBD are more likely to visit the emergency room, be admitted for medical management of their disease, and be admitted for surgery during the first five years following diagnosis.
UC and CD are chronically remitting inflammatory conditions. Both diseases are extremely variable in their presentation and course, and both have bimodal age distributions (average age of presentation 28 years with a second peak at age 50 years). The clinical presentation is more variable in CD due to the transmural nature of the condition, variability in the extent of inflammation, and extraintestinal manifestations of the disease.
Presentation of IBD varies considerably and can be subtle. The most common presentation for both diseases includes increase in stool frequency and decrease in consistency. The second most common presenting symptom is abdominal pain. In UC, this pain is cramping, located in the left lower quadrant, and improves with bowel movement, whereas in CD pain is felt in the right lower quadrant and worsens with food intake. The third most common symptom is weight loss, which is more prevalent in CD than UC, and the presenting symptom in 87% of patients with CD. Both CD and UC may exhibit extraintestinal manifestations as well (Table 163-1).
| Parallel Course | Peripheral arthritis | 5–20% |
| Erythema nodosum | 10–20% | |
| Episcleritis | 1.6–6.3% | |
| Aphthous stomatitis | 25% | |
| Pyoderma gangrenosum | 1–10% | |
| Uveitis | 0.5–9% | |
| Independent course | Ankylosing spondylitis | 5–10% |
| Sacroileitits | 3–5% | |
| Uveitits | 1.6–6.3% | |
| Primary sclerosing cholangitis | 2.4–7.5% | |
| Metabolic manifestations | Nephrolithiasis | 7–10% |
| Osteopenia, osteoporosis | 23–59% | |
| Amyloidosis | 0.07–0.9% |
Up to one-third of patients with IBD suffer from extraintestinal manifestations that can be divided into three groups. Patients with either UC or CD involving the colon frequently suffer from inflammatory conditions in the form of peripheral and axial arthropathy, erythema nodosum and pyoderma gangrenosum, episcleritis and uveitis. Inflammatory conditions involving joints, skin, and eyes usually run a course parallel to the intestinal IBD activity. Independent of the inflammatory activity in the gut are manifestations like ankylosing spondylitis, primary sclerosing cholangitis, uveitis, and sacroileitis. For these extraintestinal manifestations specific associations with human leukocyte antigens have been established. The third group of extraintestinal manifestations is due to metabolic derangements and include kidney stones, amyloidosis, and bone loss. They are parallel to intestinal activity.
Symptoms more frequently encountered with UC are left lower quadrant pain, urgency, frequency, bleeding, and mucoid discharge. Table 163-2 summarizes clinical symptoms and findings related to the extent of intestinal involvement.
| Site Involved in UC | Symptoms |
|---|---|
| Rectum | Tenesmus, rectal prolapse, bloody and mucoid discharge, constipation |
| Left-sided disease | Pain improves with bowel movement, blood mixed with stool |
| Pancolitis | Diffuse abdominal pain, abdominal distention, absent bowel sounds |
As opposed to UC with its continuous inflammation limited to the colon and occasionally the terminal ileum, CD may affect any portion of the gastrointestinal tract, from the mouth to the anus, in a discontinuous fashion, termed skip lesions. Involvement of the esophagus and stomach is uncommon, occurring in only 5% of all patients with CD. Usually, CD presents with right lower quadrant pain, nocturnal diarrhea, obstructive symptoms (nausea, vomiting, abdominal distention), fever, and weight loss (Table 163-3).
| Site Involved in CD | Symptoms |
|---|---|
| Gastroduodenum | Dyspepsia, vomiting, epigastric pain |
| Small bowel | Diarrhea, obstruction, periumbilical pain |
| Ileo-colon | Right lower quadrant pain, bleeding uncommon, obstruction |
| Colon | Tenesmus, rectal prolapse, fistula, skin maceration |
With inflammation limited to the mucosal layer of the colon, UC is clinically classified according to anatomical involvement into
- Proctitis (mainly rectal involvement), affecting the distal 10 cm to 15 cm
- Left-sided disease with inflammation extending to the descending colon
- Pancolitis, defined as disease activity extending proximally beyond the splenic flexure
In CD, transmural inflammation can affect any part of the gastrointestinal system with involvement of
- The small bowel in 80% of patients
- The ileocolon (both the ileum and the colon) in 50% of patients
- The colon in 20% of patients
CD is further defined by the inflammatory process as inflammatory, fibrostenotic, or fistulizing.
Pathophysiology
IBD manifests due to an inappropriate immune response that occurs in genetically susceptible individuals as the result of a complex interaction among environmental and microbial factors and the intestinal immune system. Incidence and prevalence of IBD are two- to four-fold and two- to nine-fold higher in Ashkenazi Jews than the nonJewish populations, respectively. A positive family history can be established in 10–20% of CD cases, which is higher in comparison to UC.
Thus far, more than 30 novel genetic IBD-susceptible gene loci have been identified and the number is growing rapidly. However, while several promising associations between IBD and gene variants have been identified and replicated effectively, genotyping is not currently standard diagnostic procedure.
Smoking has a dose-dependent beneficial effect on disease activity in UC, but not CD. More exsmokers develop UC as compared with current smokers. This is in stark contrast to CD, as smoking has a detrimental effect on disease activity. Smoking is associated with clinical recurrence of CD, and a shorter time to clinical recurrence. Both a positive family history of IBD and smoking at the time of the initial ileocolic resection are associated with an increased risk of a second ileocolic resection. Following surgically-induced remission, the time to clinical relapse for smokers was shorter (130 weeks versus 234 weeks in nonsmokers, P < 0.001). In comparison to nonsmoking patients with CD, smokers have a 2.5- and 2-fold increased risk of surgical and clinical recurrence, respectively. Therefore, aggressive strategies for smoking cessation are warranted.
Differential Diagnosis
Multiple conditions may mimic an IBD flare (Table 163-4). In-hospital acquisition of Clostridium difficile is a major concern in all patients, but especially patients with IBD. C difficile colitis is associated with a significant health care burden in hospitalized IBD patients, carries a higher mortality risk (28%), and is associated with higher resource utilization. Infection with C difficile can occur at any point in the IBD patient’s hospital admission, and the prevalence is highest among patients with UC (37.5 per 1,000 patients), followed by CD (10.9 per 1,000 patients), and lowest among patients admitted for nongastrointestinal etiologies (4.5 per 1,000 patients). Clinical deterioration in an IBD patient who has required multiple days of hospitalization should raise the suspicion for C difficile. The diagnosis is established by, preferably, three stool samples tested for toxin A and B produced by C difficile (Table 163-4).
| Disease | Diagnostic Clues |
|---|---|
| Irritable bowel syndrome | No red flags, like weight loss, bloody diarrhea, obstruction |
| Colonic ischemia | Presence of vascular diseases |
| Cytomegalovirus | Colonic IBD not improving despite therapy |
| Clostridium difficile colitis | Colonic IBD not improving despite therapy |
| Behçet disease | Mediterranean descent, skin ulcerations |
| Intestinal tuberculosis | Immigrant to the U.S. |
| Yersinia enterocolitica | Exposure to cattle, deer, and pigs |
|
Cytomegalovirus (CMV) infection of the colon is another potentially life-threatening condition that may mimic an IBD flare. Severe, therapy-refractory UC and CD should raise a high level of suspicion for this infection. High risk groups include female IBD patients presenting with fever, lymphadenopathy, splenomegaly, leukopenia, and mild hepatitis. The diagnosis is established by immunohistochemistry for viral early antigen performed on a biopsy specimen, which is significantly more sensitive than serological tests.
Diagnosis
IBD diagnosis is based on clinical history and physical examination with particular emphasis on family history and perianal examination. Currently, no single test is pathognomonic for CD or UC, and the diagnosis is supplemented by laboratory investigations, including complete blood count, liver function tests, and C-reactive protein measurement. Due to their acceptable specificity but low sensitivity, serologies for perinuclear anti-Neutrophil Cytoplasmic Antibodies (pANCA) and anti-Saccharomyces cerevisiae Antibodies (ASCA) should be used with caution in patients with a low pretest probability of IBD to prevent a delay in the correct diagnosis and consequently inappropriate treatment. Patients with CD have a positive ASCA in 50–70% of cases with a specificity of 92% if negative for pANCA. In contrast, individuals with UC are positive for pANCA in 40–80% of cases and with a specificity of 98% when found negative for ASCA. However, serologies for pANCA and ASCA might be useful for the diagnosis of indeterminate colitis.
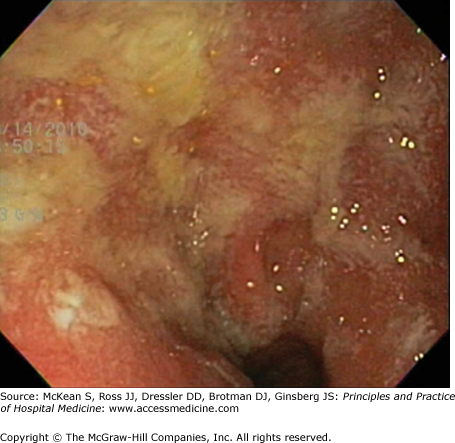
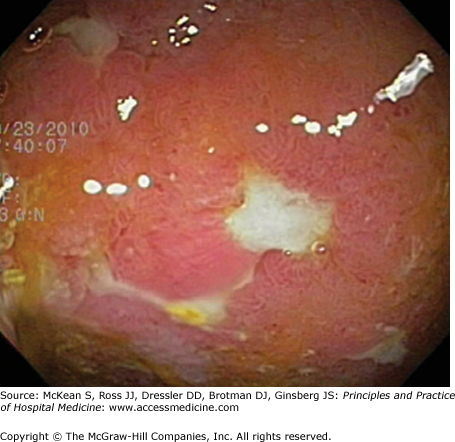
Endoscopy serves as the mainstay for IBD diagnosis, and in particular ileo-colonoscopy, with biopsies for histological examination (Table 163-5, Figures 163-1 and 163-2). Even though colonoscopy is a simple and safe procedure in patients with mild to moderate disease, it should be limited during hospitalization for a severe IBD flare to prevent perforation. Exceptions include cases of therapy-refractory disease in which limited colonoscopic intervention by an experienced operator should be considered to rule out infectious complications like C difficile colitis or CMV colitis. Ileocolonoscopy can be supplemented by wireless capsule endoscopy (WCE) for small bowel examination, but WCE should not be used during severe disease. WCE has a higher diagnostic yield than enteroclysis in the detection of CD of the small bowel, both in patients with known and newly suspected CD. Enteroclysis is a rarely performed procedure, but very helpful examination by which barium is administered through a nasogastric tube directly into the small bowel, excluding the upper gastrointestinal tract.
| UC | CD |
|---|---|
| Rectum inflamed | Rectum spared |
| Continuous inflammation | Skip lesions |
| Loss of vascularity | Aphthous ulcerations |
| Mucosal granularity | Linear and serpiginous ulcerations |
| Normal ileo-cecal valve | Fistulae |
| Ulcerated terminal ileum |
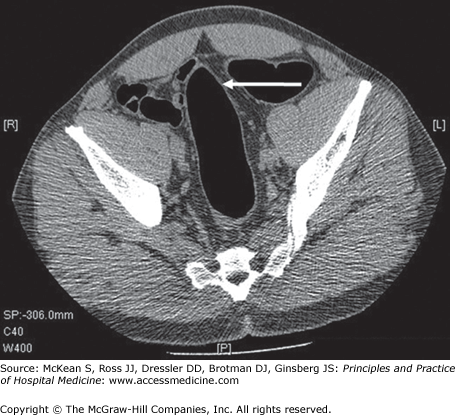
As an alternative to endoscopy, severity of inflammation can be analyzed by radiographic studies (Figures 163-3 and 163-4). Magnetic resonance (MR) enterography can reliably assess small bowel disease severity in the evaluation of ileocolonic Crohn disease. Dynamic contrast-enhanced MR imaging can help determine disease activity in perianal CD and might be helpful in selecting a subpopulation of patients who should be monitored more closely for development of more extensive disease. Alternatively, MR and CT enterography have similar sensitivities for detecting active small-bowel inflammation, and CT may have superior image quality based on study cohorts. CT was found to provide higher sensitivity in detecting enterovesical, enterocutaneous, and perianal fistulae, as well as extraluminal disease and complications, including abscesses, most commonly identified in the ischiorectal fossa, psoas muscle, and neighboring solid organs. However, CT studies raise the concern of cumulative radiation exposure, and therefore may not be a valuable option to monitor disease (Table 163-6).