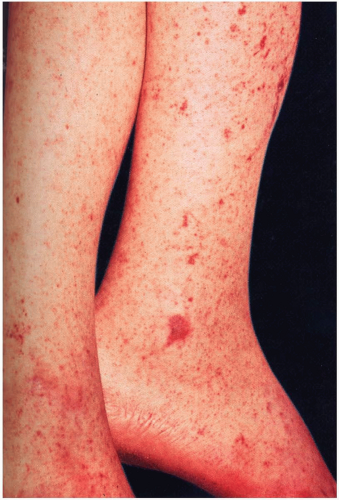
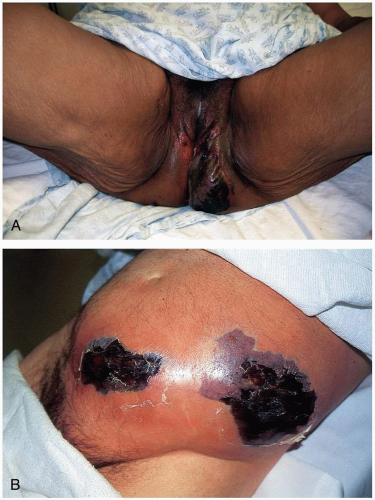
The clinical presentation of Rocky Mountain spotted fever (RMSF) is characterized by fever, headache, and a petechial rash. In the early phases, patients may develop high fever followed by a severe headache (85%). Between 50% and 60% of patients develop gastrointestinal symptoms.
1 Other early symptoms may include myalgias, sore throat, cough, abdominal pain, malaise, and anorexia.
1,
2 Two to 3 days after onset of the illness, patients develop the characteristic rash on the ankles and wrists, initially resembling pinpoint macules. The macules quickly become petechial in nature, spread to the palms and soles, and then move centrally to involve the trunk. In severe cases, this diffuse petechial rash resembles meningococcemia. The rash may coalesce, and necrosis may follow in regions supplied by terminal arterioles. The rash is not always petechial in nature, and delayed presentation of the rash is possible. Absence of an exanthem is described in 10% of cases.
3 Other signs and symptoms that may develop during the course of RMSF include diarrhea, hepatosplenomegaly, edema, lymphadenopathy, and conjunctivitis.