FIG. 5.1 Chain of infection. (Courtesy the Department of Infection Control and Epidemiology at the University of Michigan Hospitals and Health Centers, Ann Arbor, MI.)
Communicable Diseases of Concern
Every institution should have a method of identifying patients who pose a public health risk to others, and each perianesthesia unit needs to be aware of the process used for this identification. Certain communicable diseases should be clearly documented in the patient’s medical record, and the patient should be placed into appropriate patient precautions or isolation per institutional policy to minimize the infectious risks to others. Infectious diseases that warrant immediate attention include, but are not limited to, bacterial meningitis (or meningitis of unknown infectious etiology), measles (rubeola), mumps, rubella, chickenpox, tuberculosis, and severe acute respiratory syndrome. Each state in the United States publishes its own reportable disease listing that stipulates reporting requirements. Typically, the physician or the infection control department reports diseases to the local public health department. Communication is essential between the patient, health care provider, reporting department in the institution, and public health department to ensure that the chain of infection is broken.
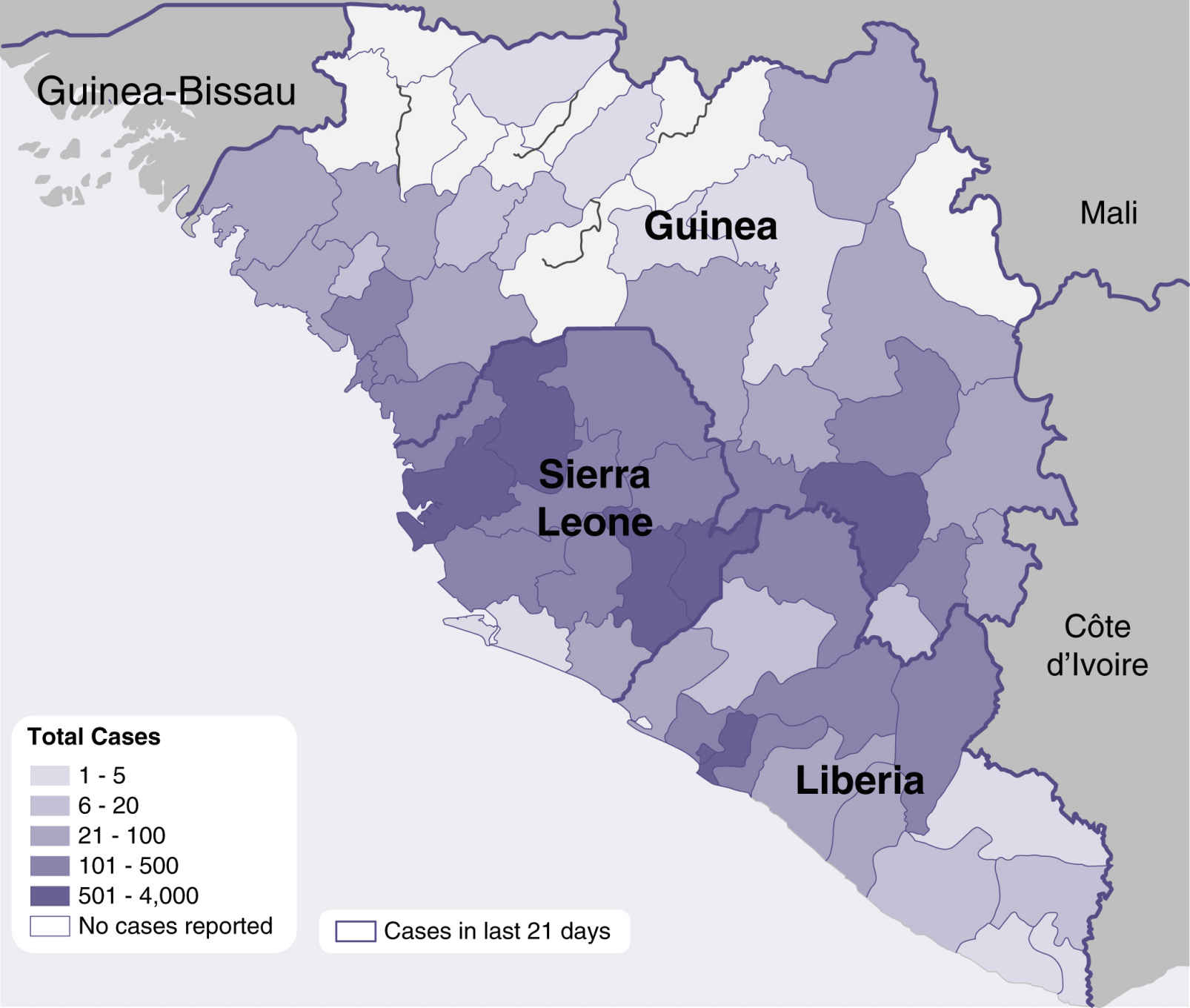
FIG. 5.2 Ebola outbreak distribution map of West Africa, 2014. (From Centers for Disease Control and Prevention (website) www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/distribution-map.html.)
The travel history of a patient is essential to the intake process to ensure knowledge and awareness of any potential exposure they may have to a communicable disease outside the country. Travel may expose an individual to diseases that are difficult to treat and/or causing outbreaks in other countries. Accurate information is essential to identify if a patient is potentially contagious with a special pathogen, which would be an uncommon or rarely seen infection in the United States.
No disease brought this need more to the forefront than Ebola. The Ebola virus causes Ebola virus disease (EVD), an invasive and often fatal infection. EVD caused an extensive outbreak in West Africa in 2014 to 2015 primarily affecting three countries (Fig. 5.2). It infected 28,601 people and caused 11,300 deaths.20 Hospitals in the United States had rarely if ever treated patients infected with Ebola and thus were not prepared with the infection control strategies necessary to contain it. A worldwide response was necessary, which required learning new clinical and infection prevention practices, as well as new PPE recommendations and products. Training needs were significant, and product shortages ensued. If a hospital had an Ebola patient who required emergency surgery, it became a complex clinical, moral, and ethical decision if they were to have the surgical procedure performed at all. Elective surgeries would be postponed until the patient was considered no longer infectious. The risks of exposure of surgical personnel and other patients, versus the benefit to the Ebola patient, would have to be carefully weighed. Decisions about where to perform the surgery were also essential (e.g., in the patient’s room versus an operating room). Ultimately, it would be unlikely that an Ebola patient would be taken into the perianesthesia environment due to the high risk this would present to other surgical patients and the surgical team.
Antibiotic-Resistant Organisms
In addition to the communicable diseases that pose an infectious risk to others, antibiotic-resistant organisms pose a significant threat to others if not properly controlled and managed. Aside from the cross-transmission risks, these organisms also cause an increase in lengths of stay, costs, and mortality rates.21–25 The most common organisms include MRSA and VRE. Certain strains of S. aureus also have intermediate susceptibility or are resistant to vancomycin (i.e., vancomycin-intermediate S. aureus [VISA], vancomycin-resistant S. aureus [VRSA]). In addition to the gram-positive organisms are certain gram-negative bacteria, including those that produce ESBLs and others resistant to multiple classes of antibiotics. Examples of resistant gram-negative bacteria include E. coli, Klebsiella pneumoniae, and Acinetobacter baumannii, and organisms such as Stenotrophomonas maltophilia.
Because of the differences in the pathogens, the diseases they cause, their routes of transmission, the patients they infect, and the intensity of patient care activities, each institution needs to have tailored infection control strategies. The perianesthesia personnel should be familiar with the epidemiology of the institution’s antibiotic-resistant organisms. This information can typically be obtained from the microbiology department or infection control department.
Patients who are vulnerable to colonization and infection include those with severe disease, especially those with compromised host defenses from underlying medical conditions; those who recently underwent surgery; or those with indwelling medical devices (e.g., urinary catheters, endotracheal tubes). Hospitalized patients, especially patients in the intensive care unit (ICU), tend to have more risk factors than nonhospitalized patients and have the highest infection rates. Increasing numbers of infections with MDROs also have been reported to occur outside the ICU.26
Ample epidemiologic evidence suggests that MDROs are carried from one person to another via the hands of HCWs. Hands are easily contaminated during the process of care giving or from contact with environmental surfaces in close proximity to the patient. The latter is especially important when patients have diarrhea, and the reservoir of the MDRO is the gastrointestinal tract. Studies of poor compliance with hand hygiene policies and glove use indicate a greater likelihood that HCWs will transmit MDROs to other patients. Thus strategies to increase and monitor adherence to policies are important components of MDRO control programs.21
An institutional control program for MDRO includes administrative support, judicious use of antimicrobials, surveillance (routine and enhanced), Standard and contact Precautions, environmental measures, education, and decolonization.
Isolation and Precautions
The CDC Hospital Infection Control Practices Advisory Committee (HICPAC) has a published guideline outlining recommendations for isolation precautions in hospitals. Two tiers of HICPAC isolation precautions exist. The first is referred to as Standard Precautions, and the second is precautions designed only for the care of specified patients. These additional transmission-based precautions are for patients known or suspected to be infected by epidemiologically important pathogens spread via airborne or droplet transmission or via contact with dry skin or contaminated surfaces.
Standard Precautions
Standard Precautions apply to all patients who receive care in hospitals, regardless of diagnosis or presumed infection status. See earlier explanation of Standard Precautions.
Transmission-Based Precautions
Transmission-based precautions are designed for patients who need additional precautions above and beyond Standard Precautions to interrupt transmission of the infectious organism. The three types of transmission-based precautions are airborne precautions, droplet precautions, and contact precautions. These precautions may be combined for diseases that have multiple routes of transmission. When used either singularly or in combination, they are to be used in addition to Standard Precautions.
Airborne precautions are designed to reduce the risk of airborne transmission of infectious agents. Airborne transmission occurs with dissemination of either airborne droplet nuclei (evaporated droplets 5 μm or smaller in size that may remain suspended in the air for long periods of time) or dust particles that contain the infectious agent. Microorganisms carried in this manner can be dispersed widely by air currents and may become inhaled by or deposited on a susceptible host within the same room or over a longer distance from the source patient, depending on environmental factors. Therefore, negative-pressure isolation rooms are necessary for these patients. If such a room is not available, the patient should be given a mask to wear and segregated away from other patients. Prompt transfer of such patients to negative-pressure isolation rooms should be undertaken to minimize the risk of transmission. During transport, the patient should be masked. Personnel who transport the patient should not be masked. Examples of diseases that require airborne precautions include pulmonary tuberculosis, chickenpox, and disseminated zoster (shingles). Personnel who care for patients in airborne precautions must wear respiratory protection (N95 respirator) when entering the room of a patient with known or suspected infectious pulmonary tuberculosis.23,24 Susceptible persons should not enter the room of patients known or suspected to have measles (rubeola) or varicella (chickenpox) if other immune caregivers are available. If susceptible persons must enter the room of a patient known or suspected to have measles (rubeola) or varicella, they should wear respiratory protection (N95 respirator).24 Persons immune to measles or varicella need not wear respiratory protection.
Droplet precautions are designed to reduce the risk of droplet transmission of infectious agents. Droplet transmission involves contact of the conjunctivae or the mucous membranes of the nose or mouth of a susceptible person with large-particle droplets (larger than 5 μm) that contain microorganisms generated from an infected person. Droplets are generated from the source person, primarily during coughing, sneezing, or talking, and while performing certain procedures such as suctioning and bronchoscopy. Transmission via large-particle droplets necessitates close contact because droplets do not remain suspended in the air and generally travel only short distances, usually 3 feet or less, through the air. Because droplets do not remain suspended in the air, a negative-pressure room is not needed to prevent droplet transmission. HCWs should wear gowns, gloves, masks, and eye protection when within 3 feet of an infected patient. Because the environment can play a role in harboring contamination, prompt cleaning of visible contamination should be performed. In addition, multiple-patient care items (e.g., blood pressure cuffs, stethoscopes) should be disinfected before use on another patient. Hands can become contaminated through holes in gloves or during removal of PPE and therefore should be promptly washed after patient care and removal of PPE. During patient transportation, the patient wearing a mask minimizes patient dispersal of droplets.
Contact precautions are designed to reduce the risk of transmission of infectious organisms with direct or indirect contact. Direct-contact transmission involves skin-to-skin contact and physical transfer of microorganisms to a susceptible person from an infected or colonized person, such as when personnel turn patients, empty drainage bags, or perform other patient care activities that require physical contact. Indirect-contact transmission involves contact of a susceptible person with a contaminated intermediate object, usually inanimate, in the patient’s environment. Examples of diseases that require contact precautions include VRE, MRSA, C. difficile, lice, and scabies. Health care workers should wear disposable gowns and gloves when providing direct care to patients in contact precautions. As in droplet precautions, a clean environment is critical to minimize transmission of disease and should be promptly and thoroughly disinfected if contaminated and on patient discharge from the area. Handwashing is of paramount importance in interrupting the spread of infections from patients in contact precautions and should be practiced diligently. Patients requiring contact precautions can be safely transported in a wheelchair or stretcher without any special requirements. However, a clean barrier (e.g., sheet, gown) should be placed between them and the transport vehicle, which should be disinfected before use with another patient. Health care workers should avoid wearing dirty gowns and gloves during transport to avoid contamination of public spaces.
A synopsis of the types of precautions and the patients who need the precautions is listed in the 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings.19 Although prospective identification of all patients who need these enhanced precautions is not possible, certain clinical syndromes and conditions carry a sufficiently high risk to warrant the empiric addition of enhanced precautions while a more definitive diagnosis is pursued. A listing of such conditions and the recommended precautions in addition to Standard Precautions is beyond the scope of this chapter but can be referred to in the Guideline for Isolation Precautions.
Patients who are immunocompromised vary in their susceptibility to health care–associated infections, depending on the severity and duration of immunosuppression. They generally are at increased risk for bacterial, fungal, parasitic, and viral infections from both endogenous (own flora) and exogenous (external) sources. The use of Standard Precautions for all patients and transmission-based precautions for specified patients, as recommended in this guideline, should greatly reduce the acquisition of pathogens by these patients from other patients, the environment, or equipment.
Prevention of Health Care–Associated Infections
According to the CDC, on any given day, approximately 1 in 25 patients in a hospital will have an HAI. These infections are referred to as nosocomial infections or as health care–associated infections (the more recent terminology), or HAI. These adverse events affect approximately 2 million patients each year in the United States, result in approximately 90,000 deaths, and add an estimated $4.5 to $5.7 billion per year to the costs of patient care.25
Most HAIs (in descending order of frequency) are caused from pneumonia, surgical site infections (SSIs), urinary tract infections, and bloodstream infections. Other infections are also present, such as gastrointestinal illness. The perianesthesia nurse can play a major role in preventing all these infections in the surgical population by adhering to time-honored practices such as asepsis and recommended evidence-based care practices. Published guidelines and compendiums are available from the CDC and other organizations on prevention of surgical site infections, intravascular catheter infections, urinary tract infections, and pneumonia.
One area of surgical patient quality improvement in which perianesthesia nurses played an active role was the Surgical Care Improvement Project (SCIP) originated by the Centers for Medicare and Medicaid Services (CMS) in 2003 and retired on December 31, 2015. This project was a national quality partnership of organizations focused on improvement of surgical care with the goal of reducing surgical complications. Partners in SCIP believe that a meaningful reduction in surgical complications depended on surgeons, anesthesia providers, nurses, pharmacists, infection control professionals, and hospital executives working together to intensify their commitment to making surgical care improvement a priority.27 SCIP identified seven processes or outcome measures related to infection prevention. They are listed in Box 5.1. Although there is no longer a requirement to submit these measures to CMS, they are still evidence-based and represent best practice.
The CDC estimates that approximately 500,000 SSIs occur annually in the United States.26 Patients who have SSIs are up to 60% more likely to spend time in an ICU, are fivefold more likely to be readmitted to the hospital, and have twice the mortality rate compared with patients without an SSI.28 A targeted process to reduce SSIs is with the appropriate antimicrobial prophylaxis administration process because, despite evidence of effectiveness of antimicrobials to prevent SSIs, previous studies have shown inappropriate timing, selection, and excess duration of administration of antimicrobial prophylaxis. Patients should be given parenteral antimicrobial prophylaxis (if indicated) within 1 hour before the surgical incision. They should also be given a prophylactic antimicrobial agent consistent with currently published guidelines as well as the institution’s antimicrobial policies.29
An additional measure within SCIP to prevent SSI is glucose control. The measure was limited to specific patient populations undergoing surgery, consistent with evidence-based medicine. The fourth SCIP CMS infection indicator addressed patients for cardiac surgery and the postoperative serum glucose. This recommendation was in part the result of studies such as Latham and colleagues who concluded that diabetes (odds ratio [OR], 2.76; p < 0.001) and postoperative hyperglycemia (OR, 2.02; p = 0.007) were independently associated with development of SSIs.30 SCIP indicator number 10 was related to maintaining normothermia in the surgical patient. It is based on a study by Kurz and colleagues,31 who reported SSIs in 18 of 96 patients undergoing colorectal surgery who were hypothermic (19%) but in only 6 of 104 patients who were normothermic (6%; p = 0.009).31
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








