KEY POINTS
1. On the day of surgery, most cardiovascular medications should be administered orally, with the exceptions of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and (selectively) diuretic agents.
2. The presence of a large-bore intravenous (IV) catheter and an arterial catheter prior to induction of anesthesia is recommended for most cardiac surgical patients. Central venous access can typically await induction of anesthesia and endotracheal intubation.
3. Prior to induction of anesthesia, critical emergency drugs should be prepared for immediate administration. Practices vary, but these drugs should include a vasopressor, vasodilator, inotrope, b-adrenergic blocker, and heparin.
4. Induction of anesthesia can be achieved using many different approaches. The most important underlying principle is suppression of the stress response while avoiding hypotension.
5. Hemodynamically tenuous patients likely will not tolerate traditional induction doses of propofol or thiopental, but even drugs typically unassociated with hypotension can cause hypotension in cardiac surgical patients as a result of anesthetic-induced reduction in sympathetic tone, synergistic circulatory depression when some induction drugs are given in combination (e.g., midazolam and fentanyl), and/or initiation of positive pressure ventilation.
6. Opioids suppress the stress response in a dose-related manner until a maximum effect is reached at an approximate dose-equivalency of 8 mg/kg of fentanyl.
7. With appropriate topicalization, sedation, and systemic stress response suppression (e.g., b-adrenergic blockers), awake intubation can be safely accomplished in cardiac patients with difficult airways.
8. Etomidate offers hemodynamic stability during induction, but also suppresses adrenal cortical function for approximately 24 h.
9. Muscle relaxant timing is important during induction, i.e., early enough to avert opioid-induced chest wall rigidity but late enough to avoid awake paralysis.

POTENT INHALATIONAL AGENTS INDUCE VASODILATION and some myocardial depression, but can be safely used during induction especially to complement IV agents such as etomidate and fentanyl.
Induction of anesthesia in a cardiac patient is more than a simple transition from an awake to a stable anesthetic state. Considering all aspects of the patient’s cardiac condition allows selection of an anesthetic that best accommodates the patient’s current cardiac status and medications. No single agent or technique can guarantee hemodynamic stability. Hemodynamic change with induction can be attributed to the patient’s pathophysiology and to a reduction in sympathetic tone potentially causing vasodilation, cardiac depression, and relative hypovolemia.
I. Premedication
A. Just as the patient’s chronic medications can mostly be used to advantage, so can premedication be an integral component of the anesthetic technique.
1
B. With rare exception, chronic cardiac medications should be administered orally preoperatively on the day of surgery with as little water as possible.
1. Some clinicians prefer to withhold diuretics on the morning of surgery, which seems reasonable.
2. ACE inhibitors and angiotensin receptor blockers have been associated with hypotension following induction of anesthesia (and perhaps during separation from cardiopulmonary bypass). Although somewhat controversial, we prefer to discontinue the patient’s usual dose of these drugs 1 day (24 h) before surgery in order to reduce the risk for hypotension and for acute kidney injury, which has been associated with ACE inhibitor use in cardiac surgery [1].
II. Preinduction period.
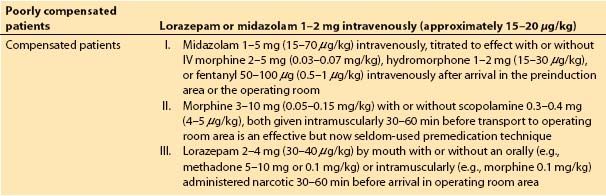
Unstable patients (e.g., critical aortic stenosis, congestive heart failure) probably should not be premedicated before they reach a preanesthetic holding area that permits observation by an anesthesia caregiver, and we perceive that few practitioners currently administer sedatives or opioids prior to the patient’s arrival in such an area. Some examples of premedication regimens are shown in Table 6.1, but monitoring catheters, such as arterial and central venous “lines,” ideally should be placed under the influence of premedication, such as IV midazolam (if not after induction of anesthesia).
Table 6.1 Anesthetic premedication for cardiac surgical patients
A. Basic monitors and supplemental oxygen are important to initiate before giving supplemental sedation (if needed) and placing invasive monitors.
1. Electrocardiogram
2. Noninvasive blood pressure (BP)
3. Pulse oximeter
B. Invasive monitors can be useful during induction [2], so some clinicians choose to place central “access” catheters, such as a central venous catheter or pulmonary artery (PA) catheter, before induction of anesthesia. It appears that most anesthesiologists defer central line placement until after anesthetic induction, which is an equally acceptable approach that does not appear to affect patient outcomes. In patients with low cardiac outputs, onset time for anesthetic induction drugs and for vasoactive drugs can be noticeably delayed when this approach is chosen. It appears that most anesthesiologists prefer to place the arterial catheter before inducing anesthesia, as do we, but some prefer to await induction of anesthesia for this intervention as well.
2
1. In emergency situations, it may be necessary to proceed with anesthetic induction before placing invasive monitors. Examples include:
a. Ruptured or rupturing thoracic aortic aneurysm
b. Cardiac tamponade
c. Ventricular rupture
2. In these situations, if a large-bore IV catheter is already present, opening the chest is far more important than PA or central venous pressure catheter measurements. It is highly desirable to initiate intra-arterial BP monitoring before anesthetic induction in such cases. If the anesthesiologist is busy stabilizing the patient and preparing for induction, the surgery team can be asked to assist with radial or femoral arterial line placement under local anesthesia.
C. Clinical tips in preparing for cardiac anesthesia
1. Emergency drugs that may be needed can be prepared as dilute bolus doses in syringes or as IV or syringe infusion pumps attached to a peripheral IV or to the infusion port of the PA (or some other central venous) catheter via a manifold.
2. The drugs selected for anesthesia depend on the patient’s condition and the preferences of the anesthesiologist.
a. Commonly fentanyl or sufentanil, and less commonly remifentanil, are selected as opioids.
b. Among the potent inhalational agents, isoflurane probably should be selected for economy, desflurane for rapid titratability, and sevoflurane for inhalational induction for an airway that is suspected to be difficult (as a judgment-based alternative to awake intubation).
c. IV amnestic agents may include benzodiazepines such as midazolam, lorazepam, or diazepam, or any of the traditional induction agents described below.
d. For muscle relaxation, succinylcholine (or no muscle relaxant) is suggested for a suspect airway; pancuronium for economy and low heart rates (HRs); vecuronium, rocuronium, or cisatracurium for hemodynamic blandness; and cisatracurium in the case of liver or renal failure. Note that even moderate to severe impairment in renal or hepatic function need not contraindicate any muscle relaxant per se, but may instead simply merit adjustments in dosing frequency.
3. Specific cardiovascular medications to have available (asterisk indicates probable need to have the drug drawn up and ready for administration) before surgery:
a. Anticholinergic: Atropine* (preferred over glycopyrrolate for faster onset)
3
b. Inotrope: Epinephrine*, dobutamine, or dopamine as infusions; epinephrine also in a syringe for bolus administration
c. Phosphodiesterase III inhibitor: Milrinone
d. Calcium chloride
e. Ephedrine* as a bolus (mixed vasopressor/inotrope)
f. Vasopressors
(1) Phenylephrine as a 50- to 100-μg bolus*, or as an infusion, or norepinephrine
(2) Vasopressin (for vascular collapse and resuscitation, especially after left ventricular assist device placement)
g. Vasodilators: Nitroglycerin*, nicardipine, and nitroprusside
h. Antiarrhythmics and antitachycardic agents
(1) Adenosine
(2) Esmolol*, metoprolol, or propranolol
(3) Diltiazem or verapamil
(4) Lidocaine*
(5) Amiodarone
(6) Magnesium
i. Anticoagulation and its reversal
(1) Heparin*
(2) Protamine
j. To be most prepared, at least one inotrope, one vasopressor, and one vasodilator should be set up and connected in a pump that is preprogrammed and ready to use. Similarly, syringes should be prepared for bolus administration of at least one vasopressor, inotrope, vasodilator, and probably a β-adrenergic blocker as well.
k. A custom-built IV pole with a built-in electrical outlet and the capability for attachment of multiple IV infusion or syringe pumps should be available. In the case of syringe pumps, battery operation is acceptable if there is a reliable mechanism for recharging the syringe pumps before each procedure.
D. Last-minute checks: Immediately before anesthetic induction, the following points should be considered:
1. Reassessment of the patient’s overall cardiopulmonary and airway status
2. Integrity of breathing circuit and immediate availability of suction
3. Availability of blood for transfusion
4. Proximity of a surgeon or a senior resident
5. Any special endotracheal tube needs (double-lumen, bronchial blocker)
6. Immediate availability of emergency cardiac drugs
III. Induction.
The cardiac anesthesiologist must often induce a patient who under normal circumstances would not receive a general anesthetic. Objectives include:
A. Attenuation of hemodynamic responses to laryngoscopy and surgery without undue hypotension
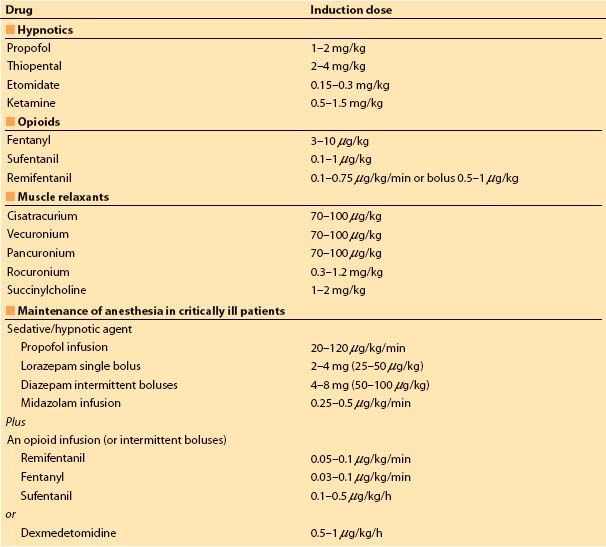
1. Conservative drug amounts, as the anesthetic requirement rapidly becomes relatively minor during surgical skin preparation and draping (exception: high-dose opioid induction technique) (Table 6.2)
4
Table 6.2 Recommended induction doses
2. Use drug onset time and interactions to advantage.
3. Adapt induction drug doses to physical status of patient.
B. Guiding principles for anesthetic induction include:
1. Modifications of techniques as new knowledge are gained. For example, trace the history of sufentanil as used during anesthetic induction.
a. In the 1980s, the recommended induction dose of sufentanil was as high as 25 mg/kg.
b. In the 1990s, recommendations changed to 6 to 10 mg/kg.
c. In the new millennium, as little as 0.1 mg/kg is used.
d. Combinations of sufentanil or fentanyl with etomidate and muscle relaxants exemplify efficient induction techniques.
2. Physiologic issues
a. Hypovolemia, which is often difficult to assess, is frequently caused by diuretics and prolonged nothing by mouth (NPO) status.
(1) This is difficult to assess because of the absence of preoperative urine output documentation or left ventricular (LV) preload assessment.
(2) Most cardiac patients do not tolerate more than 10% depletion of intravascular volume without hemodynamic compromise.
(3) Tachycardia and vasoconstriction, which are useful compensatory mechanisms in normal individuals, may be deleterious in cardiac patients or may be impaired or even precluded as a result of the patient’s chronic cardiovascular drug regimen.
(4) Anesthetic drugs may impair appropriate hemodynamic responses [3].
5
(a) Propofol and thiopental reduce BP by inducing venodilation with peripheral pooling of blood, decreasing sympathetic tone to decrease systemic vascular resistance (SVR), and depressing myocardial contractility.
(b) In decreasing order of circulatory depression, propofol is most depressant, thiopental slightly less so, midazolam is intermediate, and etomidate is the least depressant.
(5) The most physiologically efficient method of combating hypovolemia is to augment intravascular volume in the preinduction period using balanced salt solutions, being careful not to overdo this in patients with mitral valve lesions or congestive heart failure. The presence or absence of susceptibility to congestive heart failure may be subtle in patients with LV diastolic dysfunction. In general, the trend has been toward more conservative use of fluids in the precardiopulmonary bypass period in order to minimize hemodilution and the need for RBC transfusion. At times, this occurs at the expense of circulatory support with vasopressors. This philosophy is sound as long as sufficient cardiac output is maintained to perfuse the vital organs and (arguably) is most safely applied if one can measure cardiac output to ensure an adequate cardiac index (under anesthesia, probably greater than or equal to 1.8 L/min/m2). The initiation of an inotropic infusion (low-dose epinephrine 0.01 to 0.03 mcg/kg/min or dopamine 1 to 3 mcg/kg/min) in such patients sometimes helps to maintain a stable perfusion pressure and cardiac output during the preinduction period.
3. Pharmacodynamic issues. With the exception of ketamine, which can stimulate the cardiovascular system, all anesthetics decrease BP by some combination of removing sympathetic tone, directly decreasing SVR, directly depressing the myocardium, increasing venous pooling (reducing venous return), or inducing bradycardia.
Important individual drug characteristics to be considered:
a. In critically ill patients, ketamine can decrease BP because depletion of catecholamines may lead to an inability of indirect central nervous system-mediated sympathomimetic effects to counterbalance its direct negative inotropic effects.
b. The conflict between the need to attenuate the hemodynamic response to intubation and other noxious stimuli without overdosing can be illustrated by propofol.
(1) Propofol may induce hypotension if used for induction, yet a small dose may not suppress the hypertensive response to laryngoscopy.
(2) In combination with other drugs: After an induction dose of propofol, systolic BP fell an average of 28 mm Hg when no fentanyl was administered, whereas it fell 53 mm Hg when 2 mg/kg of fentanyl was administered. However, the hemodynamic response to intubation was decreased in proportion to the preinduction dose of fentanyl [4].
(3) Propofol can be given in small increments (0.5 to 1 mg/kg) judging from the patient’s physical status and can be used with a small well-timed dose of opioid (e.g., 1 to 3 mg/kg of fentanyl 1 to 2 min preceding propofol).
c. The principle of using the relationship between the plasma drug concentration and the bioeffector site onset (biophase or Ke0) must be considered, such that the maximal effects of both the opioid and hypnotic are used to best advantage.
(1) The mean onset time for peak effect of propofol is 2.9 min and for fentanyl is 6.4 min. Ideally, endotracheal intubation should be performed at the peak concentration of both drugs and after optimal muscle relaxation has been achieved. This may require a second dose or a continuous infusion of ultrashort-acting induction agents such as propofol, because the muscle relaxant onset time may occur after the peak effect of the bolus dose of the induction agent has dissipated from rapid redistribution.
6
d. Increasing the opioid dose beyond 8 μg/kg of fentanyl, 0.75 μg/kg of sufentanil, or 1.2 μg/kg/min of remifentanil does not further attenuate the stress response (increased BP and HR) to intubation.
e. Depth of anesthesia provided by propofol or other sedative–hypnotic agents does not determine the degree of stress-response suppression; rather, the central nervous system level of opioid analgesia tends to do so.
f. Reducing the doses of anesthetic drugs is often the safest way to induce critically ill patients.
g. For a hemodynamically stable patient, one might prefer a hemodynamically bland muscle relaxant except for patients with a baseline HR less than 50 beats/min or with valvular regurgitation, where pancuronium can still be useful.
h. Induction drugs are administered most efficiently through a central venous catheter, such as the side-port introducer, or an infusion port in a PA catheter.
i. Muscle relaxants are given early in the sequence. Onset time is important to consider. This can be defined for most agents in the context of ED95, or the average dose required to induce 95% suppression of the twitch response. For most nondepolarizing neuromuscular blockers, the time to achieve maximum twitch suppression at a dose of 1 × ED95 is 3 to 7 min. Use of 2 × or 3 × ED95 reduces onset time to 1.5 to 3 min, and to as low as 1 to 1.5 min for 3 × ED95 with rocuronium (0.9 mg/kg).
(1) Succinylcholine (1 to 2 mg/kg) can be used to reduce onset time of neuromuscular blockade to 1 to 1.5 min.
j. An example of a drug combination that combines rapid onset (intubating conditions in 1 to 2 min) and good suppression of the stress response to laryngoscopy is remifentanil 1 mg/kg, etomidate 0.2 mg/kg, and succinylcholine 1.5 mg/kg, all administered as a simultaneous bolus.
k. High-dose opioid induction techniques:
(1) Achieved popularity in the 1970s with morphine (1 to 2 mg/kg) or fentanyl (50 to 100 mg/kg) because of the combination of excellent stress–response suppression and hemodynamic stability.
(2) Sufentanil (10 to 25 mg/kg) rose to popularity for the same reasons in the 1980s.
(3) This technique lost favor in the 1990s because of long postoperative intubation times, but it is still potentially useful for high-risk patients who will require overnight mechanical ventilation regardless of the anesthetic technique chosen.
(4) Because of the marked vagotonic effects of bolus high-dose opioids, pancuronium nicely complements this technique and should be given early in the induction sequence to minimize chest wall rigidity.
(5) These doses of fentanyl and sufentanil can be given as a bolus or over 3 to 5 min. Morphine must be given slowly (5 to 10 mg/min) to avoid hypotension. A recent study indicated that morphine may still have a place in cardiac anesthesia, as it reduced the degree of postoperative pain and the incidence of postoperative fever [5].
(6) Beware of hypotension if hypnotics are given simultaneously and of inadequate amnesia if they are not.
C. Anticipated difficult intubation. The cardiac patient with a difficult airway imposes a conflict between our instincts to preserve and protect the airway and those to avoid hemodynamic stress. Concerns about loss of airway control supersede those about hemodynamic stimulation, yet both airway safety and hemodynamic stability can be achieved with awake intubation. Suggestions for accomplishing “awake” endotracheal intubation (by any of several techniques) while still preventing potentially deleterious hemodynamics follow:
7
1. Adequate airway anesthesia either greatly attenuates or prevents the hemodynamic stress of endotracheal intubation. Specific techniques that may prove helpful include:
a. Nebulized 4% lidocaine for 15 or more minutes prior to airway.
b. Topical sprays such as Cetacaine or 10% lidocaine, although toxicity can result from either of these agents if used to excess.
c. Nerve blocks: Glossopharyngeal and superior laryngeal blocks can anesthetize the pharynx down to the level of the vocal cords.
d. Transcricoid or transtracheal injection of 4% lidocaine can suppress the cough reflex, as can injection of 4% lidocaine via the suction or injection port of a fiberoptic bronchoscope upon reaching the vocal cords and trachea.
2. Low to moderate levels of sedation can facilitate patient cooperation and may induce amnesia:
a. Midazolam titrated to effect.
b. Opioids in low doses, but beware of the combined sedative and respiratory depressant effects of opioids combined with sedative/hypnotics, such as midazolam.
c. Conservative infusion doses of propofol (e.g., 10 to 40 mg/kg) can be helpful and may reduce the gag and cough reflex modestly, but can also result in dysphoria or airway obstruction.
d. Some practitioners select dexmedetomidine for this purpose, whereas others remain unimpressed with its efficacy or desirability. Favorable aspects include sedation (without reliable amnesia!), tendency to decrease HR and BP, and possibly modest airway reflex suppression. Typically the dose for awake intubation would be 1 mg/kg administered intravenously over approximately 10 min.
3. Adjuncts that may help prevent or treat hypertension or tachycardia:
a. β-Adrenergic blockers (e.g., esmolol bolus [0.25 to 1 mg/kg] or infusion [100 to 300 mg/kg/min])
b. Vasodilators (e.g., nicardipine 500 to 750 mg or nitroglycerin 50 to 100 mg bolus, or continuous infusion titrated to effect)
c. Mixed adrenergic blocker: IV labetalol titrated to effect (bolus dose typically 10 to 20 mg every 5 to 10 min)
4. Be prepared to proceed with a gentle IV induction once endotracheal intubation has been achieved. If the preparations above have been successful, the patient will tolerate the presence of the endotracheal tube without coughing or distress, which reduces the urgency to proceed with induction.
IV. Opioids
A. Basic structures and opioid receptors
1. Rigid interlocked molecules of the morphine group known as pentacyclides and flexible molecules of phenylpiperidine rings, such as fentanyl
2. There are three opioid receptors (m, k, and d) with subgroups.
a. Opioid receptors are g-protein–coupled receptors.
B. Properties of opioids. Analgesia is more than the relief of pain or of the conscious perception of a nociceptive stimulus. A noxious stimulus can affect an ostensibly unconscious (and unparalyzed) person as demonstrated by movement in the form of a withdrawal reflex. The stimulus can produce increased autonomic activity. Narcotics in general are poor hypnotics and cannot be counted upon to induce amnesia.
C. Induction pharmacokinetics:
1. Pharmacokinetics are similar among three modern synthetic opioids (fentanyl, sufentanil, and alfentanil) with a few differences [6].
a. All have a three-compartment model.
b. Ninety-eight percent of fentanyl is redistributed from the plasma in the first hour.
c. Brain levels parallel plasma levels with a lag of 5 min.
d. Fentanyl has a large volume of distribution, which can limit hepatic access. However, the liver will clear all the fentanyl it gets.
e. Sufentanil is 7 to 10 times more potent than fentanyl. It has a higher pKa and only 20% is ionized.
f. Sufentanil is half as lipid soluble as fentanyl and is more tightly bound to receptors. It has a lower volume of distribution and a faster recovery time.
g. Alfentanil, typically administered as a continuous infusion, is less potent than fentanyl and shorter acting than sufentanil, but has been largely superseded by remifentanil (see below).
2. Remifentanil pharmacokinetics:
a. Remifentanil has a unique pharmacokinetic profile, as it is subject to widespread extrahepatic hydrolysis by nonspecific tissue and blood esterases.
b. It has an onset time of 1 min and a recovery time of 9 to 20 min. These properties make it very advantageous when there is variation of surgical stimulus or a desire for early postoperative extubation. The anesthesiologist can give as much as he or she feels is needed without impeding rapid recovery.
c. Careful provision of postoperative pain control is essential, as remifentanil-induced analgesia dissipates rapidly after the infusion is terminated.
V. Other IV anesthetic agents
A. Etomidate
1. Etomidate, a very useful induction agent for cardiac patients, is 10 times more potent than propofol, with a recommended dose range of 0.15 to 0.3 mg/kg.
2. It is reliable at achieving hypnosis, especially when combined with an opioid. Administering the primary dose just after giving an opioid may attenuate myoclonus, which sometimes occurs as a result of subcortical disinhibition.
3. Etomidate reaches the brain in 1 min.
4. There may be an increased incidence of epileptiform activity in patients with known epileptic seizure disorders.
5. A dose of etomidate typically produces a 10% to 15% decrease in mean arterial pressure and SVR and a 3% to 4% increase in HR and cardiac output.
6. Importantly, stroke volume, left ventricular end-diastolic volume (LVEDV), and contractility remain unchanged in normovolemic patients.
7. Etomidate can be used to anesthetize heart transplant patients, because it preserves myocardial contractility better than any induction technique other than a high-dose opioid induction.
8
8. Although traditional induction doses of etomidate and opioids given individually most often preserve hemodynamics, when they are given together hypotension may ensue.
9. Since even a single dose of etomidate has been shown to induce significant adrenal suppression for more than 24 h (not associated with increased vasopressor requirement), it should be used with caution in high-risk cardiac surgical patients or followed by glucocorticoid supplementation for 24 to 48 h [7].
B. Propofol
1. A normal induction dose of 2 mg/kg will drop BP 15% to 40%.
2. Because propofol resets the baroreceptor reflex, lower BP does not increase HR.
3. There are significant reductions in SVR, cardiac index, stroke volume, and LV stroke work index.
4. There is direct myocardial depression at doses above 0.75 mg/kg.
5. Propofol should be titrated according to the patient’s age, weight, and individual need and ideally injected into a central vein, thereby allowing the smallest dose to be used effectively and avoiding pain on injection.
6. Propofol’s metabolic clearance is 10 times faster than that of thiopental.
7. There is extensive redistribution and movement from the central compartment to a peripheral one, which enables rapid recovery.
8. Unless one wishes to decrease BP, use of propofol for induction does not have an advantage over etomidate. Because it has direct myocardial depressant effects and easily induces hypotension, propofol should be used with caution or reserved for use in hemodynamically stable cardiac patients with good ventricular function.
C. Thiopental
1. Thiopental is currently not available in the United States.
2. It has a rapid onset and can be used safely in hemodynamically stable patients.
3. Rapid redistribution to highly perfused tissues causes cessation of thiopental’s effects.
4. Cardiovascular effects
a. Predominantly venous pooling and resultant decreased cardiac preload.
b. Myocardial depressant above 2 mg/kg.
c. Increases HR by activating baroreceptor reflex.
d. In patients who have low cardiac output, a greater proportion of the drug dose goes to the brain and myocardium; thus, a smaller amount of thiopental has a larger effect.
e. Overall, there is a dose-related negative inotropic effect from a decrease in calcium influx.
D. Midazolam
1. Midazolam is a good premedicant but is difficult to titrate to a minimum effective dose for induction because of a large variation in the required dose and a relatively slow onset time to peak CNS effect of 3 to 7 min. A typical induction dose is 0.1 to 0.2 mg/kg.
2. It is an effective amnestic, and this constitutes its appeal.
3. The hypotensive effect from an induction dose is similar to or less than that for thiopental, and it is dose related.
4. In patients who have high cardiac filling pressures, midazolam seems to mimic low-dose nitroglycerin by reducing filling pressures.
5. The addition of opioids produces a supra-additive hypotensive effect.
E. Lorazepam and Diazepam
9
1. Lorazepam is a very potent benzodiazepine (approximately 1.5 times as potent as midazolam), and diazepam is approximately half as potent as midazolam.
2. Because of its potency, lorazepam produces anxiolytic, sedative, and amnestic effects in lower doses and with fewer side effects than midazolam. Diazepam’s cardiovascular effects are comparable to those for midazolam, i.e., generally modest preload and afterload reduction that appears to be enhanced in the presence of potent opioids such as fentanyl, sufentanil, and remifentanil.
3. Lorazepam is useful in sick cardiac patients when only small amounts of drugs are desired. Both lorazepam and diazepam can complement high-dose opioid inductions as long as the slower onset times are understood and accommodated.
4. If rapid recovery is expected, as in minimally invasive direct coronary artery bypass surgery, lorazepam is a poor choice because of its relatively long clinical action (typically several hours). Diazepam in moderate to high doses (greater than 0.15 mg/kg for most patients) can exhibit a prolonged action as well, and it has an active metabolite. Diazepam’s clinical offset is disproportionately prolonged in elderly patients when compared to lorazepam or midazolam.
5. Onset times are relatively slow (lorazepam peaks in 5 to 10 min, diazepam is slightly faster) in the context of induction of anesthesia, but are acceptable in the context of IV sedation for “line” placement before induction of anesthesia.
F. Ketamine
1. Ketamine produces a unique cataleptic trance known as dissociative anesthesia.
2. It is extensively redistributed and eliminated.
3. Bioavailability on IV injection is 97% and 2 mg/kg produces unconsciousness in 20 to 60 s.
4. Ketamine induces significant increases in HR, mean arterial pressure, and plasma epinephrine levels. This sympathetic nervous system stimulation is centrally mediated.
5. Ketamine may be advantageous in hypovolemia, major hemorrhage, or cardiac tamponade.
6. It allows humane obtundation of the hemodynamically unstable patient, giving the surgeon an opportunity to rapidly intervene and correct a life-threatening problem (e.g., cardiac tamponade). In these situations, skin preparation should be performed before induction.
7. The hemodynamic stimulatory effect of ketamine depends on the presence of a robust myocardium and sympathetic reserve. In the absence of either, hypotension may ensue from myocardial depression [8].
8. Coronary blood flow may not be sufficient to meet the increased oxygen demands induced by sympathetic stimulation.
9. Ketamine should be avoided in patients with elevated intracranial pressure.
10. The S+ isomer produces much longer periods of hypnosis and analgesia, and less postanesthetic stimulation. This compound, currently available in some European countries, may become available in the United States.
11. Ketamine is very useful for patients who have experienced severe acute blood loss.
VI. Inhalational agents
A. Hemodynamic effects. Similar but generally modest levels of myocardial depression occur with all three popular inhalational agents, isoflurane, desflurane, and sevoflurane. Serious consequences may occur in patients with congestive heart failure, however, as a narrow range of anesthetic concentrations may be tolerated by the compromised myocardium. The predominant hemodynamic effect of these three agents is dose-dependent vasodilation, hence reducing BP and SVR [9]. All three agents also induce a dose-dependent reflex tachycardia that can be attenuated or prevented by b-adrenergic blockers or opioids.
B. Desflurane is uniquely titratable for induction of anesthesia because of its rapid onset and offset, which remarkably matches that of a remifentanil infusion. Because of its pungent aroma, however, desflurane is poorly tolerated unless it is preceded by an IV induction.
C. Sevoflurane has a much more pleasant aroma, suitable for inhalational induction, offers hemodynamic stability in most induction situations, and has an onset time only slightly slower than that of desflurane.
D. Isoflurane, like desflurane, has a pungent aroma, and it is best introduced after an IV induction.
E. Nitrous oxide is seldom used during anesthetic induction in cardiac surgical patients, but it is generally safe to use for induction with the probable exception of patients with markedly increased pulmonary vascular resistance.
F. Clinical use. Whereas clinically significant brain concentrations (greater than or equal to 1 × minimal anesthetic concentration [MAC]) can be attained with desflurane and sevoflurane in 2 to 4 min, generally lower concentrations are achieved over the same time frame with isoflurane. Consequently, desflurane and sevoflurane are more likely to reach concentrations consistent with stress–response suppression (generally 1.3 to 1.5 times MAC) during a customary induction period than isoflurane. One potential drawback to desflurane is sympathetic stimulation when the inspired concentration is increased rapidly, perhaps owing to its airway irritant effects. Any of these inhalation agents can be used during induction as a complement to an IV induction. Desflurane can be useful in cardiac anesthesia not as much because of its rapid offset as because of its rapid onset.
VII. Muscle relaxants
A. A suspected difficult intubation precludes giving the patient a neuromuscular blocker before achieving intubation unless one is highly confident that mask ventilation will succeed and that an emergency alternative airway (e.g., laryngeal mask, fiberoptic intubation) can also succeed.
B. Succinylcholine still has the fastest onset and offset of all muscle relaxants.
C. Significant b-adrenergic blockade and high-dose opioid induction are potential indications for otherwise obsolescent pancuronium, as its vagolytic effects tend to counter the vagotonia and bradycardia induced by higher doses of opioids.
D. Intermediate-duration agents: Cisatracurium, rocuronium, and vecuronium are hemodynamically bland.
1. If hepatic or renal failure exists, cisatracurium appears to be the wisest choice.
E. Timing of the administration of the muscle relaxant is important.
1. Laryngoscopy should await optimal relaxation (see Section I.B.3.i). Early administration obviates opioid-induced truncal rigidity, which may impair mask ventilation and result in systemic oxygen desaturation, yet one should also ensure amnesia with a sedative-hypnotic agent before administering the muscle relaxant.
VIII. Applications of old drugs in sick patients.
One busy cardiac surgery center blends the principles of careful patient assessment, cautious dosing, and fiscal restraint by commonly choosing the following preinduction sequence:
A. Patient arrives in anesthetic preinduction area or operating room.
1. A large-bore (16-gauge or larger) IV catheter is placed.
2. Light premedication is administered, typically midazolam 1 to 2 mg IV.
3. A 20-gauge radial or brachial arterial catheter is placed using local anesthesia.
B. IV induction of anesthesia proceeds using the following:
1. Fentanyl 250 to 500 mg in consideration of the patient’s size and hemodynamic stability.
2. Etomidate 0.15 to 0.2 mg/kg with the same consideration.
3. After ensuring that mask ventilation can be accomplished, succinylcholine 1 to 2 mg/kg is administered.
4. Endotracheal intubation is accomplished.
C. After endotracheal intubation, the next steps are as follows:
1. Placement of central venous access (e.g., double-lumen CVP or 9 French introducer with single-lumen IV catheter or a PA catheter placed through the introducer for hemodynamic monitoring)
2. As hemodynamics permit, careful initiation of isoflurane is administered at 0.5 to 1 MAC with titration to BP and bispectral index.
3. If needed, IV phenylephrine is titrated to support BP.
4. Upon recovery from succinylcholine, transition to vecuronium (initial dose approximately 0.03 to 0.05 mg/kg, subsequent doses 0.01 to 0.02 mg/kg) for maintenance of neuromuscular blockade. For longer cases when fast tracking is not anticipated, pancuronium (30 to 50 mg/kg) is an acceptable alternative.
IX. Inhalational Induction in very sick patients.
This technique is a good alternative to gently induce very sick patients with low ejection fraction undergoing LVAD placement or heart transplantation. The technique is recommended only in patients who clearly have empty stomachs in order to avoid the potential for aspiration, as the induction period is prolonged:
A. Patient is in the operating room, monitors placed.
B. Large bore (16 gauge or larger) IV catheter is placed.
C. Light premedication with midazolam 1 to 2 mg IV.
D. Twenty-gauge radial arterial catheter is placed using local anesthetic (2% lidocaine).
E. An inotropic infusion (usually epinephrine or dopamine) is connected to the peripheral IV, programmed and ready to go.
F. Start preoxygenation and 2% sevoflurane, maintain 2% during entire induction period, and decrease it only if hemodynamic instability ensues.
G. Administer fentanyl typically 150 to 500 mg in divided doses, depending on the patient size and age.
H. Consider administering additional midazolam boluses usually up to 5 mg total, or increasing the inspired concentration of sevoflurane to 3% to 4% if hemodynamics remain stable.
I. Upon loss of consciousness, after testing the airway, administer 0.6 to 0.9 mg/kg rocuronium to allow for rapid intubation.
J. When mask ventilating, hyperventilate with small tidal volumes to decrease PA pressure, and to avoid intrathoracic overinflation that may increase pulmonary vascular resistance and decrease venous return. For similar reasons, avoid positive-end-expiratory pressure and be aware of the possibility of “breath-stacking” in patients with reactive airways disease or chronic obstructive pulmonary disease (COPD). The latter patients may require prolonged expiratory time.
X. Immediate postinduction period.
After induction and intubation, several different techniques may be used for maintenance of anesthesia. First priorities, however, are to assess the airway (confirm endotracheal tube location via end-tidal CO2 and auscultation), assess hemodynamic stability, and respond appropriately to any problems identified.
A. A low-dose continuous infusion of the opioid used for induction or of remifentanil (0.1 mg/kg/min) can be implemented.
B. Consider adding an inhalational agent for maintenance of anesthesia and amnesia.
C. Continuous infusion or intermittent bolus doses of a sedative–hypnotic agent (e.g., midazolam) can be initiated if no potent inhalational agent is used.
D. A propofol infusion may be useful in hemodynamically robust patients.
E. Some clinicians prefer a dexmedetomidine infusion for its augmentation of analgesia and tendency to avoid hypertension, although its offset time is relatively long (15 min or more), it can induce hypertension at onset, and bradycardia and hypotension may occur.
F. The treatment of postinduction hypotension deserves mention. There is a tendency to administer phenylephrine boluses of 100 to 200 mg as a “knee-jerk” response to hypotension, when at times either rapid volume infusion, an alternative vasoactive drug, or both may be more appropriate.
1. If the heart appears empty based on filling pressures, echocardiography findings, cardiac output measurement, or respiratory variation in systolic pressure, then rapid administration of crystalloid or colloid is appropriate.
2. If the induction has used drugs most likely to reduce SVR and preload without affecting myocardial contractility (e.g., midazolam or etomidate with an opioid and a muscle relaxant), then phenylephrine is appropriate. If the need appears likely to be sustained because of a lengthy interval to surgical incision, then consider a continuous phenylephrine infusion of 0.1 to 1 mg/kg/min.
3. If the HR is low or if there is a strong possibility of myocardial depression as well (e.g., propofol was used for induction or >0.5 MAC of a volatile agent is in use), then consider using a bolus of ephedrine (5 to 15 mg) or epinephrine (10 to 25 mg).
G. A simple technique used daily and varied in dosage according to the physical status of the patient probably provides the most consistent results for most clinicians.
REFERENCES
1. Sun J-Z, Cao L-H, Liu H. ACE inhibitors in cardiac surgery: Current studies and controversies. Hypertension Res. 2011;34: 15–22.
2. Reich DL, Kaplan JA. Hemodynamic monitoring. In: Kaplan JA, ed. Cardiac Anesthesia. 4th ed. Philadelphia, PA: WB Saunders; 1999:321–358.
3. Harrison NL, Sear JW. Barbiturates, etomidate, propofol, ketamine, and steroids. In: Evers AS, Maze M, eds. Anesthetic Pharmacology: Physiologic Principles and Clinical Practice. Philadelphia, PA: Churchill Livingstone; 2004:395–416.
4. Billard V, Moulla F, Bourgain JL, et al. Hemodynamic response to induction and intubation: Propofol/fentanyl interaction. Anesthesiology. 1994;81:1384–1393.
5. Murphy GS, Szokil JW, Marymont JH, et al. Morphine-based cardiac anesthesia provides superior early recovery compared with fentanyl in elective cardiac surgery patients. Anesth Analg. 2009;109:311–319.
6. Bovill JG. Opioids. In: Bovill JG, Howie MB, eds. Clinical Pharmacology for Anaesthetists. London: WB Saunders; 1999: 87–102.
7. Morel J, Salard M, Castelain C, et al. Hemodynamic consequences of etomidate administration in elective cardiac surgery: A randomized double blinded study. Br J Anesth. 2011;107(4):503–509.
8. Schuttler J, Zsigmond EK, White PF. Ketamine and its isomers. In: White PF, ed. Textbook of Intravenous Anesthesia. Philadelphia, PA: Williams & Wilkins; 1997:171–188.
9. Pagel PS, Warltier DC. Anesthetics and left ventricular function. In: Warltier DC, ed. Ventricular Function, a Society of Cardiovascular Anesthesiologists Monograph. Baltimore, MD: Williams & Wilkins; 1995:213–252.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








